
In immunology,autoimmunity is the system of immune responses of an organism against its own healthy cells,tissues and other normal body constituents. Any disease resulting from this type of immune response is termed an "autoimmune disease". Prominent examples include celiac disease,post-infectious IBS,diabetes mellitus type 1,Henoch–Schönlein purpura (HSP) sarcoidosis,systemic lupus erythematosus (SLE),Sjögren syndrome,eosinophilic granulomatosis with polyangiitis,Hashimoto's thyroiditis,Graves' disease,idiopathic thrombocytopenic purpura,Addison's disease,rheumatoid arthritis (RA),ankylosing spondylitis,polymyositis (PM),dermatomyositis (DM),and multiple sclerosis (MS). Autoimmune diseases are very often treated with steroids.

A cytotoxic T cell (also known as TC,cytotoxic T lymphocyte,CTL,T-killer cell,cytolytic T cell,CD8+ T-cell or killer T cell) is a T lymphocyte (a type of white blood cell) that kills cancer cells,cells that are infected by intracellular pathogens (such as viruses or bacteria),or cells that are damaged in other ways.

In immunology,a memory B cell (MBC) is a type of B lymphocyte that forms part of the adaptive immune system. These cells develop within germinal centers of the secondary lymphoid organs. Memory B cells circulate in the blood stream in a quiescent state,sometimes for decades. Their function is to memorize the characteristics of the antigen that activated their parent B cell during initial infection such that if the memory B cell later encounters the same antigen,it triggers an accelerated and robust secondary immune response. Memory B cells have B cell receptors (BCRs) on their cell membrane,identical to the one on their parent cell,that allow them to recognize antigen and mount a specific antibody response.
In immunology,affinity maturation is the process by which TFH cell-activated B cells produce antibodies with increased affinity for antigen during the course of an immune response. With repeated exposures to the same antigen,a host will produce antibodies of successively greater affinities. A secondary response can elicit antibodies with several fold greater affinity than in a primary response. Affinity maturation primarily occurs on membrane immunoglobulin of germinal center B cells and as a direct result of somatic hypermutation (SHM) and selection by TFH cells.

Cytotoxic T-lymphocyte associated protein 4,(CTLA-4) also known as CD152,is a protein receptor that functions as an immune checkpoint and downregulates immune responses. CTLA-4 is constitutively expressed in regulatory T cells but only upregulated in conventional T cells after activation –a phenomenon which is particularly notable in cancers. It acts as an "off" switch when bound to CD80 or CD86 on the surface of antigen-presenting cells. It is encoded by the gene CTLA4 in humans.

CD23,also known as Fc epsilon RII,or FcεRII,is the "low-affinity" receptor for IgE,an antibody isotype involved in allergy and resistance to parasites,and is important in regulation of IgE levels. Unlike many of the antibody receptors,CD23 is a C-type lectin. It is found on mature B cells,activated macrophages,eosinophils,follicular dendritic cells,and platelets.

Germinal centers or germinal centres (GCs) are transiently formed structures within B cell zone (follicles) in secondary lymphoid organs –lymph nodes,ileal Peyer's patches,and the spleen –where mature B cells are activated,proliferate,differentiate,and mutate their antibody genes during a normal immune response;most of the germinal center B cells (BGC) are removed by tingible body macrophages. There are several key differences between naive B cells and GC B cells,including level of proliferative activity,size,metabolic activity and energy production. The B cells develop dynamically after the activation of follicular B cells by T-dependent antigen. The initiation of germinal center formation involves the interaction between B and T cells in the interfollicular area of the lymph node,CD40-CD40L ligation,NF-kB signaling and expression of IRF4 and BCL6.

X-linked severe combined immunodeficiency (X-SCID) is an immunodeficiency disorder in which the body produces very few T cells and NK cells.

CD154,also called CD40 ligand or CD40L,is a protein that is primarily expressed on activated T cells and is a member of the TNF superfamily of molecules. It binds to CD40 on antigen-presenting cells (APC),which leads to many effects depending on the target cell type. In total CD40L has three binding partners:CD40,α5β1 integrin and integrin αIIbβ3. CD154 acts as a costimulatory molecule and is particularly important on a subset of T cells called T follicular helper cells. On TFH cells,CD154 promotes B cell maturation and function by engaging CD40 on the B cell surface and therefore facilitating cell-cell communication. A defect in this gene results in an inability to undergo immunoglobulin class switching and is associated with hyper IgM syndrome. Absence of CD154 also stops the formation of germinal centers and therefore prohibiting antibody affinity maturation,an important process in the adaptive immune system.
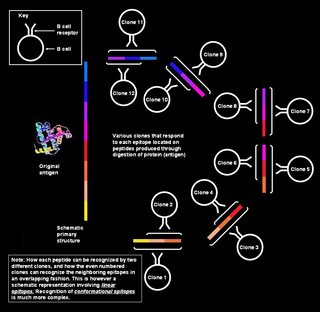
Polyclonal B cell response is a natural mode of immune response exhibited by the adaptive immune system of mammals. It ensures that a single antigen is recognized and attacked through its overlapping parts,called epitopes,by multiple clones of B cell.

B-lymphocyte antigen CD19,also known as CD19 molecule,B-Lymphocyte Surface Antigen B4,T-Cell Surface Antigen Leu-12 and CVID3 is a transmembrane protein that in humans is encoded by the gene CD19. In humans,CD19 is expressed in all B lineage cells. Contrary to some early doubts,human plasma cells do express CD19,as confirmed by others. CD19 plays two major roles in human B cells:on the one hand,it acts as an adaptor protein to recruit cytoplasmic signaling proteins to the membrane;on the other,it works within the CD19/CD21 complex to decrease the threshold for B cell receptor signaling pathways. Due to its presence on all B cells,it is a biomarker for B lymphocyte development,lymphoma diagnosis and can be utilized as a target for leukemia immunotherapies.

CD137,a member of the tumor necrosis factor (TNF) receptor family,is a type 1 transmembrane protein,expressed on surfaces of leukocytes and non-immune cells. Its alternative names are tumor necrosis factor receptor superfamily member 9 (TNFRSF9),4-1BB, and induced by lymphocyte activation (ILA). It is of interest to immunologists as a co-stimulatory immune checkpoint molecule,and as a potential target in cancer immunotherapy.

Intercellular adhesion molecule 2 (ICAM2),also known as CD102,is a human gene,and the protein resulting from it.

CD69 is a human transmembrane C-Type lectin protein encoded by the CD69 gene. It is an early activation marker that is expressed in hematopoietic stem cells,T cells,and many other cell types in the immune system. It is also implicated in T cell differentiation as well as lymphocyte retention in lymphoid organs.

G-protein coupled receptor 183 also known as Epstein-Barr virus-induced G-protein coupled receptor 2 (EBI2) is a protein (GPCR) expressed on the surface of some immune cells,namely B cells and T cells;in humans it is encoded by the GPR183 gene. Expression of EBI2 is one critical mediator of immune cell localization within lymph nodes,responsible in part for the coordination of B cell,T cell,and dendritic cell movement and interaction following antigen exposure. EBI2 is a receptor for oxysterols. The most potent activator is 7α,25-dihydroxycholesterol (7α,25-OHC),with other oxysterols exhibiting varying affinities for the receptor. Oxysterol gradients drive chemotaxis,attracting the EBI2-expressing cells to locations of high ligand concentration. The GPR183 gene was identified due to its upregulation during Epstein-Barr virus infection of the Burkitt's lymphoma cell line BL41,hence its name:EBI2.

Interferon regulatory factor 4 (IRF4) also known as MUM1 is a protein that in humans is encoded by the IRF4 gene,. IRF4 functions as a key regulatory transcription factor in the development of human immune cells. The expression of IRF4 is essential for the differentiation of T lymphocytes and B lymphocytes as well as certain myeloid cells. Dysregulation of the IRF4 gene can result in IRF4 functioning either as an oncogene or a tumor-suppressor,depending on the context of the modification.

The TCIRG1 gene encodes for the V-type proton ATPase (V-ATPase) 116 kDa subunit a isoform 3 enzyme.
X-linked lymphoproliferative disease is a lymphoproliferative disorder,usually caused by SH2DIA gene mutations in males. XLP-positive individuals experience immune system deficiencies that render them unable to effectively respond to the Epstein-Barr virus (EBV),a common virus in humans that typically induces mild symptoms or infectious mononucleosis (IM) in patients. There are two currently known variations of the disorder,known as XLP1 and XLP2. XLP1 is estimated to occur in approximately one in every million males,while XLP2 is rarer,estimated to occur in one of every five million males. Due to therapies such as chemotherapy and stem cell transplants,the survival rate of XLP1 has increased dramatically since its discovery in the 1970s.

Max Dale Cooper,is an American immunologist and a professor at the Department of Pathology and Laboratory Medicine and the Emory Vaccine Center of Emory University School of Medicine. He is known for characterizing T cells and B cells.
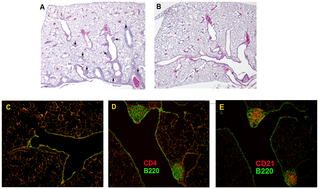
Bronchus-associated lymphoid tissue (BALT) is a tertiary lymphoid structure. It is a part of mucosa-associated lymphoid tissue (MALT),and it consists of lymphoid follicles in the lungs and bronchus. BALT is an effective priming site of the mucosal and systemic immune responses.

















