Gossypol is a natural phenol derived from the cotton plant. Gossypol is a phenolic aldehyde that permeates cells and acts as an inhibitor for several dehydrogenase enzymes. It is a yellow pigment. The structure exhibits axial chirality, with the two enantiomers having different biochemical properties.

Catechin is a flavan-3-ol, a type of natural phenol and antioxidant. It is a plant secondary metabolite. It belongs to the group of flavan-3-ols, part of the chemical family of flavonoids.

Gingerol, properly as [6]-gingerol, is a phenol phytochemical compound found in fresh ginger that activates spice receptors on the tongue. Molecularly, gingerol is a relative of capsaicin and piperine, the compounds which are alkaloids, though the bioactive pathways are unconnected. It is normally found as a pungent yellow oil in the ginger rhizome, but can also form a low-melting crystalline solid. This chemical compound is found in all members of the Zingiberaceae family plant and is high in concentrations in the grains of paradise as well as an African Ginger species.

Caffeic acid is an organic compound that is classified as a hydroxycinnamic acid. This yellow solid consists of both phenolic and acrylic functional groups. It is found in all plants because it is a key intermediate in the biosynthesis of lignin, one of the principal components of woody plant biomass and its residues.

Coeloglossum is a genus of flowering plants in the orchid family Orchidaceae. It has long been considered to have only one species, Coeloglossum viride, the frog orchid. Some recent classifications regard Coeloglossum as part of the larger genus, Dactylorhiza, so that C. viride becomes Dactylorhiza viridis. Other sources continue to keep Coeloglossum viride separate.

Gastrodia elata is a saprophytic perennial herb in the family Orchidaceae. It is found in Nepal, Bhutan, India, Japan, North Korea, Siberia, Taiwan, and China.

Scutellarin is a flavone, a type of phenolic chemical compound. It can be found in Scutellaria barbata and S. lateriflora. The determination of the structure of scutellarin took Guido Goldschmiedt many years: after the first publication on that topic in 1901, only in 1910 he managed to obtain enough starting material for more detailed studies.
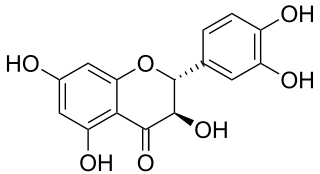
Taxifolin (5,7,3',4'-flavan-on-ol), also known as dihydroquercetin, belongs to the subclass flavanonols in the flavonoids, which in turn is a class of polyphenols.
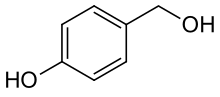
4-Hydroxybenzaldehyde is one of the three isomers of hydroxybenzaldehyde. It can be found in the orchids Gastrodia elata and Galeola faberi. It is also found in vanilla, also an orchid species.
Cāng zhú, also known as black atractylodes rhizome or Rhizoma Atractylodes, is a Chinese herbal medicine. It is the dried rhizome of Atractylodes lancea (Thunb.) DC., Atractylodes chinensis (DC.) Koidz, or certain other local species including Atractylodes japonica Koidz. The medicine is distinguished from bái zhú, which is typically cultivated, whereas cāng zhú more often tends to be collected from the wild. It is believed that the distinction between cāng zhú and bái zhú emerged in relatively modern times; a single drug "zhú" described in the Shen nong ben cao jing probably included many Atractylodes species.

In biochemistry, naturally occurring phenols refers to phenol functional group that is found in natural products. Phenolic compounds are produced by plants and microorganisms. Organisms sometimes synthesize phenolic compounds in response to ecological pressures such as pathogen and insect attack, UV radiation and wounding. As they are present in food consumed in human diets and in plants used in traditional medicine of several cultures, their role in human health and disease is a subject of research. Some phenols are germicidal and are used in formulating disinfectants.

Gastrodin is a chemical compound which is the glucoside of gastrodigenin. It has been isolated from the orchid Gastrodia elata and from the rhizome of Galeola faberi. It can also be produced by biotransformation of 4-hydroxybenzaldehyde by Datura tatula cell cultures.
2,4-Bis(4-hydroxybenzyl)phenol is a phenolic compound produced by the saprophytic orchid Gastrodia elata and by the myco-heterotroph orchid Galeola faberi.
Galeola faberi is an orchid species in the genus Galeola found in central and southern China, as well as in Nepal, the eastern Himalayas, Vietnam and Sumatra.
Gastrol is a phenolic compound produced by the saprophytic orchid Gastrodia elata.

The diarylheptanoids are a relatively small class of plant secondary metabolites. Diarylheptanoids consist of two aromatic rings joined by a seven carbons chain (heptane) and having various substituents. They can be classified into linear (curcuminoids) and cyclic diarylheptanoids. The best known member is curcumin, which is isolated from turmeric and is known as food coloring E100. Some other Curcuma species, such as Curcuma comosa also produce diarylheptanoids.

Habenariol is a phenolic compound found in the semi-aquatic orchid Habenaria repens. It acts as a feeding deterrent against the freshwater crayfish Procambarus clarkii.

Thienorphine is a very potent, extremely long-acting, orally-active opioid analgesic with mixed agonist–antagonist properties which was developed by the Beijing Institute of Pharmacology and Toxicology as a potential treatment for opioid dependence. It is a high-affinity, balanced ligand of the μ-, δ-, and κ-opioid receptors, behaving as a partial agonist of the μ- and κ-opioid receptors and as an antagonist of the δ-opioid receptor. It also possesses relatively low affinity for the nociceptin receptor, where it acts as an antagonist.
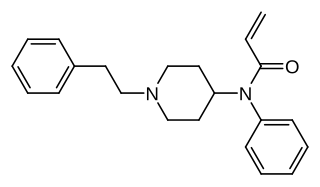
Acrylfentanyl (also known as acryloylfentanyl or Egyptenyl) is a highly potent opioid analgesic that is an analog of fentanyl and has been sold online as a designer drug. In animal studies the IC50 or half maximal inhibitory concentration for acrylfentanyl to displace naloxone is 1.4 nM, being slightly more potent than fentanyl itself (1.6 nM) as well as having a longer duration of action.

5α-Dihydrolevonorgestrel (5α-DHLNG) is an active metabolite of the progestin levonorgestrel which is formed by 5α-reductase. It has about one-third of the affinity of levonorgestrel for the progesterone receptor. In contrast to levonorgestrel, the compound has both progestogenic and antiprogestogenic activity, and hence has a selective progesterone receptor modulator-like profile of activity. This is analogous to the case of norethisterone and 5α-dihydronorethisterone. In addition to the progesterone receptor, 5α-DHLNG interacts with the androgen receptor. It has similar affinity for the androgen receptor relative to levonorgestrel, and has androgenic effects similarly to levonorgestrel and testosterone. 5α-DHLNG is further transformed into 3α,5α- and 3β,5α-THLNG, which bind weakly to the estrogen receptor and have weak estrogenic activity. These metabolites are considered to be responsible for the weak estrogenic activity of high doses of levonorgestrel.