Bioaccumulation is the gradual accumulation of substances, such as pesticides or other chemicals, in an organism. Bioaccumulation occurs when an organism absorbs a substance faster than it can be lost or eliminated by catabolism and excretion. Thus, the longer the biological half-life of a toxic substance, the greater the risk of chronic poisoning, even if environmental levels of the toxin are not very high. Bioaccumulation, for example in fish, can be predicted by models. Hypothesis for molecular size cutoff criteria for use as bioaccumulation potential indicators are not supported by data. Biotransformation can strongly modify bioaccumulation of chemicals in an organism.

Vanillin is an organic compound with the molecular formula C8H8O3. It is a phenolic aldehyde. Its functional groups include aldehyde, hydroxyl, and ether. It is the primary component of the extract of the vanilla bean. Synthetic vanillin is now used more often than natural vanilla extract as a flavoring in foods, beverages, and pharmaceuticals.

Lonicera japonica, known as Japanese honeysuckle and golden-and-silver honeysuckle, is a species of honeysuckle native to East Asia, including many parts of China. It is often grown as an ornamental plant, but has become an invasive species in a number of countries. It is used in traditional Chinese medicine.

The Adephaga are a suborder of beetles, and with more than 40,000 recorded species in 10 families, the second-largest of the four beetle suborders. Members of this suborder are collectively known as adephagans. The largest family is Carabidae which comprises most of the suborder with over 40,000 species. Adephaga also includes a variety of aquatic beetles, such as predaceous diving beetles and whirligig beetles.

Ferulic acid is a hydroxycinnamic acid derivative and a phenolic compound. It is an organic compound with the formula (CH3O)HOC6H3CH=CHCO2H. The name is derived from the genus Ferula, referring to the giant fennel (Ferula communis). Classified as a phenolic phytochemical, ferulic acid is an amber colored solid. Esters of ferulic acid are found in plant cell walls, covalently bonded to hemicellulose such as arabinoxylans. Salts and esters derived from ferulic acid are called ferulates.
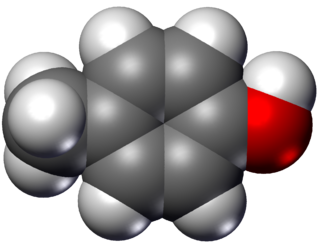
para-Cresol, also 4-methylphenol, is an organic compound with the formula CH3C6H4(OH). It is a colourless solid that is widely used intermediate in the production of other chemicals. It is a derivative of phenol and is an isomer of o-cresol and m-cresol.

Hordenine is an alkaloid of the phenethylamine class that occurs naturally in a variety of plants, taking its name from one of the most common, barley. Chemically, hordenine is the N-methyl derivative of N-methyltyramine, and the N,N-dimethyl derivative of the well-known biogenic amine tyramine, from which it is biosynthetically derived and with which it shares some pharmacological properties. As of September 2012, hordenine is widely sold as an ingredient of nutritional supplements, with the claims that it is a stimulant of the central nervous system, and has the ability to promote weight loss by enhancing metabolism. In experimental animals, given sufficiently large doses parenterally, hordenine does produce an increase in blood pressure, as well as other disturbances of the cardiovascular, respiratory, and nervous systems. These effects are generally not reproduced by oral administration of the drug in test animals, and virtually no scientific reports of the effects of hordenine in human beings have been published.

Phenanthrenoids are chemical compounds formed with a phenanthrene backbone. These compounds occur naturally in plants, although they can also be synthesized.

Triphenyl phosphate (TPhP) is the chemical compound with the formula OP(OC6H5)3. It is the simplest aromatic organophosphate. This colourless solid is the ester (triester) of phosphoric acid and phenol. It is used as a plasticizer and a fire retardant in a wide variety of settings and products.

4‑Hydroxybenzaldehyde (para‑hydroxybenzaldehyde) is an organic compound with the formula C6H4OH(CHO). Along with 2-hydroxybenzaldehyde and 3-hydroxybenzaldehyde, it is one of the three isomers of hydroxybenzaldehyde.

Phlorotannins are a type of tannins found in brown algae such as kelps and rockweeds or sargassacean species, and in a lower amount also in some red algae. Contrary to hydrolysable or condensed tannins, these compounds are oligomers of phloroglucinol (polyphloroglucinols). As they are called tannins, they have the ability to precipitate proteins. It has been noticed that some phlorotannins have the ability to oxidize and form covalent bonds with some proteins. In contrast, under similar experimental conditions three types of terrestrial tannins apparently did not form covalent complexes with proteins.
Ascidae is a family of mites in the order Mesostigmata.

Phenolic acids or phenolcarboxylic acids are phenolic compounds and types of aromatic acid compounds. Included in that class are substances containing a phenolic ring and an organic carboxylic acid function. Two important naturally occurring types of phenolic acids are hydroxybenzoic acids and hydroxycinnamic acids, which are derived from non-phenolic molecules of benzoic and cinnamic acid, respectively.

In biochemistry, naturally occurring phenols are natural products containing at least one phenol functional group. Phenolic compounds are produced by plants and microorganisms. Organisms sometimes synthesize phenolic compounds in response to ecological pressures such as pathogen and insect attack, UV radiation and wounding. As they are present in food consumed in human diets and in plants used in traditional medicine of several cultures, their role in human health and disease is a subject of research. Some phenols are germicidal and are used in formulating disinfectants.
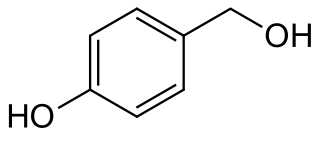
Gastrodigenin is a phenolic compound found in the rhizome of Gastrodia elata.

Dihydrostilbenoids (bibenzyls) are natural phenols formed from the dihydrostilbene (bibenzyl) backbone.

Habenaria repens, commonly called the water-spider bog orchid or the floating orchid, is an orchid species widespread across Latin America from Mexico and the West Indies south to Argentina, as well as in the Southeastern United States from Texas and Oklahoma east to Florida and the Carolinas plus an isolated population in Virginia.

Pungenin is a phenolic compound found in the needles of Blue Spruce. It is the glucoside of 3,4-dihydroxyacetophenone.
Orchid mycorrhizae are endomycorrhizal fungi which develop symbiotic relationships with the roots and seeds of plants of the family Orchidaceae. Nearly all orchids are myco-heterotrophic at some point in their life cycle. Orchid mycorrhizae are critically important during orchid germination, as an orchid seed has virtually no energy reserve and obtains its carbon from the fungal symbiont.

Freshwater salinization is the process of salty runoff contaminating freshwater ecosystems, which can harm aquatic species in certain quantities and contaminate drinking water. It is often measured by the increased amount of dissolved minerals than what is considered usual for the area being observed. Naturally occurring salinization is referred to as primary salinization; this includes rainfall, rock weathering, seawater intrusion, and aerosol deposits. Human-induced salinization is termed as secondary salinization, with the use of de-icing road salts as the most common form of runoff. Approximately 37% of the drainage in the United States has been affected by salinization in the past century. The EPA has defined two thresholds for healthy salinity levels in freshwater ecosystems: 230 mg/L Cl− for average salinity levels and 860 mg/L Cl− for acute inputs.

















