
In organic chemistry, phenols, sometimes called phenolics, are a class of chemical compounds consisting of one or more hydroxyl groups bonded directly to an aromatic hydrocarbon group. The simplest is phenol, C
6H
5OH. Phenolic compounds are classified as simple phenols or polyphenols based on the number of phenol units in the molecule.

Phenol is an aromatic organic compound with the molecular formula C6H5OH. It is a white crystalline solid that is volatile. The molecule consists of a phenyl group bonded to a hydroxy group. Mildly acidic, it requires careful handling because it can cause chemical burns.

In organic chemistry, the phenyl group, or phenyl ring, is a cyclic group of atoms with the formula C6H5, and is often represented by the symbol Ph. The phenyl group is closely related to benzene and can be viewed as a benzene ring, minus a hydrogen, which may be replaced by some other element or compound to serve as a functional group. A phenyl group has six carbon atoms bonded together in a hexagonal planar ring, five of which are bonded to individual hydrogen atoms, with the remaining carbon bonded to a substituent. Phenyl groups are commonplace in organic chemistry. Although often depicted with alternating double and single bonds, the phenyl group is chemically aromatic and has equal bond lengths between carbon atoms in the ring.

Caffeic acid is an organic compound with the formula (HO)2C6H3CH=CHCO2H. It is a polyphenol. It is a yellow solid. Structurally, it is classified as a hydroxycinnamic acid. The molecule consists of both phenolic and acrylic functional groups. It is found in all plants as an intermediate in the biosynthesis of lignin, one of the principal components of biomass and its residues. It is unrelated to caffeine.

Ferulic acid is a hydroxycinnamic acid derivative and a phenolic compound. It is an organic compound with the formula (CH3O)HOC6H3CH=CHCO2H. The name is derived from the genus Ferula, referring to the giant fennel (Ferula communis). Classified as a phenolic phytochemical, ferulic acid is an amber colored solid. Esters of ferulic acid are found in plant cell walls, covalently bonded to hemicellulose such as arabinoxylans. Salts and esters derived from ferulic acid are called ferulates.

In organic chemistry, alkyl nitrites are a group of organic compounds based upon the molecular structure R−O−N=O, where R represents an alkyl group. Formally they are alkyl esters of nitrous acid. They are distinct from nitro compounds.

The Folin–Ciocâlteu reagent (FCR) or Folin's phenol reagent or Folin–Denis reagent, is a mixture of phosphomolybdate and phosphotungstate used for the colorimetric in vitro assay of phenolic and polyphenolic antioxidants, also called the gallic acid equivalence method (GAE). It is named after Otto Folin, Vintilă Ciocâlteu, and Willey Glover Denis. The Folin-Denis reagent is prepared by mixing sodium tungstate and phosphomolybdic acid in phosphoric acid. The Folin–Ciocalteu reagent is just a modification of the Folin-Denis reagent. The modification consisted of the addition of lithium sulfate and bromine to the phosphotungstic-phosphomolybdic reagent.

Gastrodia elata is a saprophytic perennial herb in the family Orchidaceae. It is found in Nepal, Bhutan, India, Japan, Korea, Siberia, Taiwan, and China.
The Hoesch reaction or Houben–Hoesch reaction is an organic reaction in which a nitrile reacts with an arene compound to form an aryl ketone. The reaction is a type of Friedel-Crafts acylation with hydrogen chloride and a Lewis acid catalyst.

Alkylphenols are a family of organic compounds obtained by the alkylation of phenols. The term is usually reserved for commercially important propylphenol, butylphenol, amylphenol, heptylphenol, octylphenol, nonylphenol, dodecylphenol and related "long chain alkylphenols" (LCAPs). Methylphenols and ethylphenols are also alkylphenols, but they are more commonly referred to by their specific names, cresols and xylenols. Some members of this group of compounds have proven controversial.

BSPP is a compound used in scientific research which acts as a positive allosteric modulator at the GABAB receptor. It has a synergistic effect with GABAB agonists such as baclofen at GABAB autoreceptors but not heteroreceptors, suggesting it may be useful for distinguishing between these GABAB receptor subtypes.

4‑Hydroxybenzaldehyde (para‑hydroxybenzaldehyde) is an organic compound with the formula C6H4OH(CHO). Along with 2-hydroxybenzaldehyde and 3-hydroxybenzaldehyde, it is one of the three isomers of hydroxybenzaldehyde.

Theogallin is a trihydroxybenzoic acid glycoside, a type of polyphenolic compound found in tea where it has been characterised as an umami enhancing compound. The compound can also be found in Arbutus unedo fruits.

Phenolic acids or phenolcarboxylic acids are phenolic compounds and types of aromatic acid compounds. Included in that class are substances containing a phenolic ring and an organic carboxylic acid function. Two important naturally occurring types of phenolic acids are hydroxybenzoic acids and hydroxycinnamic acids, which are derived from non-phenolic molecules of benzoic and cinnamic acid, respectively.

In biochemistry, naturally occurring phenols are natural products containing at least one phenol functional group. Phenolic compounds are produced by plants and microorganisms. Organisms sometimes synthesize phenolic compounds in response to ecological pressures such as pathogen and insect attack, UV radiation and wounding. As they are present in food consumed in human diets and in plants used in traditional medicine of several cultures, their role in human health and disease is a subject of research. Some phenols are germicidal and are used in formulating disinfectants.

Gastrodin is a chemical compound which is the glucoside of gastrodigenin. It has been isolated from the orchid Gastrodia elata and from the rhizome of Galeola faberi. It can also be produced by biotransformation of 4-hydroxybenzaldehyde by Datura tatula cell cultures.

The grape reaction product is a phenolic compound explaining the disappearance of caftaric acid from grape must during processing. It is also found in aged red wines. Its enzymatic production by polyphenol oxidase is important in limiting the browning of musts, especially in white wine production. The product can be recreated in model solutions.
2,4-Bis(4-hydroxybenzyl)phenol is a phenolic compound produced by the saprophytic orchid Gastrodia elata and by the myco-heterotroph orchid Galeola faberi.
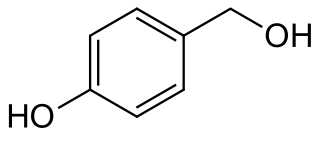
Gastrodigenin is a phenolic compound found in the rhizome of Gastrodia elata.

Drupanol is a naturally occurring phenol that has been isolated from the seeds of Psoralea drupaceae. Although drupanol is sometimes said to be the same compound as bakuchiol, the two compounds are in fact distinct; they have the same molecular formula and weight but different chemical structures and hence are structural isomers. Bakuchiol has been found to possess antiandrogenic activity in vitro.
















