
Cytarabine, also known as cytosine arabinoside (ara-C), is a chemotherapy medication used to treat acute myeloid leukemia (AML), acute lymphocytic leukemia (ALL), chronic myelogenous leukemia (CML), and non-Hodgkin's lymphoma. It is given by injection into a vein, under the skin, or into the cerebrospinal fluid. There is a liposomal formulation for which there is tentative evidence of better outcomes in lymphoma involving the meninges.
Okadaic acid, C44H68O13, is a toxin produced by several species of dinoflagellates, and is known to accumulate in both marine sponges and shellfish. One of the primary causes of diarrhetic shellfish poisoning, okadaic acid is a potent inhibitor of specific protein phosphatases and is known to have a variety of negative effects on cells. A polyketide, polyether derivative of a C38 fatty acid, okadaic acid and other members of its family have shined light upon many biological processes both with respect to dinoflagellete polyketide synthesis as well as the role of protein phosphatases in cell growth.

Malonyl-CoA is a coenzyme A derivative of malonic acid.

(+)-Discodermolide is a polyketide natural product found to stabilize microtubules. (+)-discodermolide was isolated by Gunasekera and his co-workers at the Harbor Branch Oceanographic Institute from the deep-sea sponge Discodermia dissoluta in 1990. (+)-Discodermolide was found to be a potent inhibitor of tumor cell growth in several MDR cancer cell lines. (+)-discodermolide also shows some unique characters, including a linear backbone structure, immunosuppressive properties both in vitro and in vivo, potent induction of an accelerated senescence phenotype, and synergistic antiproliferative activity in combination with paclitaxel. Discodermolide was recognized as one of the most potent natural promoters of tubulin assembly. A large number of efforts toward the total synthesis of (+)-discodermolide were directed by its interesting biological activities and extreme scarcity of natural sources. The compound supply necessary for complete clinical trials cannot be met by harvesting, isolation, and purification. As of 2005, attempts at synthesis or semi-synthesis by fermentation have proven unsuccessful. As a result, all discodermolide used in preclinical studies and clinical trials has come from large-scale total synthesis.

2-Imidazoline (Preferred IUPAC name: 4,5-dihydro-1H-imidazole) is one of three isomers of the nitrogen-containing heterocycle imidazoline, with the formula C3H6N2. The 2-imidazolines are the most common imidazolines commercially, as the ring exists in some natural products and some pharmaceuticals. They also have been examined in the context of organic synthesis, coordination chemistry, and homogeneous catalysis.

Stuart Schreiber is an American chemist who is the Morris Loeb Research Professor at Harvard University, a co-founder of the Broad Institute, Howard Hughes Medical Institute Investigator, Emeritus, and a member of the National Academy of Sciences and National Academy of Medicine. He currently leads Arena BioWorks.
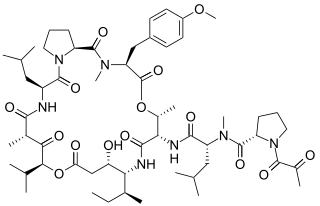
Plitidepsin is a chemical compound extracted from the ascidian Aplidium albicans. It is currently undergoing clinical trial testing. It is a member of the class of compounds known as didemnins.
A depsipeptide is a peptide in which one or more of its amide, -C(O)NHR-, groups are replaced by the corresponding ester, -C(O)OR-. Many depsipeptides have both peptide and ester linkages. Elimination of the N–H group in a peptide structure results in a decrease of H-bonding capability, which is responsible for secondary structure and folding patterns of peptides, thus inducing structural deformation of the helix and β-sheet structures. Because of decreased resonance delocalization in esters relative to amides, depsipeptides have lower rotational barriers for cis-trans isomerization and therefore they have more flexible structures than their native analogs. They are mainly found in marine and microbial natural products.

Calyculins are natural products originally isolated from the marine sponge Discodermia calyx. Calyculins have proven to be strong serine/threonine protein phosphatase inhibitors and based on this property, calyculins might be potential tumor-promoting agents.

Indolocarbazoles (ICZs) are a class of compounds that are under current study due to their potential as anti-cancer as well as antimicrobial drugs and the prospective number of derivatives and uses found from the basic backbone alone. First isolated in 1977, a wide range of structures and derivatives have been found or developed throughout the world. Due to the extensive number of structures available, this review will focus on the more important groups here while covering their occurrence, biological activity, biosynthesis, and laboratory synthesis.

Dynamic combinatorial chemistry (DCC); also known as constitutional dynamic chemistry (CDC) is a method to the generation of new molecules formed by reversible reaction of simple building blocks under thermodynamic control. The library of these reversibly interconverting building blocks is called a dynamic combinatorial library (DCL). All constituents in a DCL are in equilibrium, and their distribution is determined by their thermodynamic stability within the DCL. The interconversion of these building blocks may involve covalent or non-covalent interactions. When a DCL is exposed to an external influence, the equilibrium shifts and those components that interact with the external influence are stabilised and amplified, allowing more of the active compound to be formed.

Palau'amine is a toxic chlorinated alkaloid compound synthesized naturally by certain species of sea sponges. The name of the molecule derives from the island nation of Palau, near where the first sponge species discovered to produce it, Stylotella agminata, is found. It has since been isolated in other sponges, including Stylissa massa.
3-Alkylpyridinium (3-AP) compounds are natural chemical compounds that are found in marine sponges belonging to the order Haplosclerida. Some polymers derived from 3-APs are anticholinesterase agents and show hemolytic and cytotoxic activities. More than 70 structurally different 3-APs have been isolated from marine sponges. However, not all such sponges contain 3-AP compounds. Variation in content of 3-APs has been detected even within a single sponge species collected from different geographical area. Although 3-APs look structurally quite simple, the structure elucidation by NMR spectroscopy is complicated by the fact that most of the methylene groups in the alkyl chains show the same chemical shift. Therefore, the 3-APs are an ideal test case for a combined approach of NMR spectroscopy and mass spectrometry.

Callystatin A is a polyketide natural product from the leptomycin family of secondary metabolites. It was first isolated in 1997 from the marine sponge Callyspongia truncata which was collected from the Goto Islands in the Nagasaki Prefecture of Japan by the Kobayashi group. Since then its absolute configuration has been elucidated and callystatin A was discovered to have anti-fungal and anti-tumor activities with extreme potency against the human epidermoid carcinoma KB cells (IG50 = 10 pg/ml) and the mouse lymphocytic leukemia Ll210 cells (IG50 = 20 pg/ml).

The cortistatins are a group of steroidal alkaloids first isolated in 2006 from the marine sponge Corticium simplex. The cortistatins were first discovered in a search for naturally occurring compounds that inhibit proliferation of human umbilical vein endothelial cells (HUVECs), with cortistatin A being the most potent compound in the class.

Oroidin is a bromopyrrole alkaloid, originally isolated from marine sponges in the genus Agelas. It appears to have a wide range of biological activities, which makes Oroidin a potential drug candidate for various diseases. It also serves as chemical defense in marine sponges.

Lacking an immune system, protective shell, or mobility, sponges have developed an ability to synthesize a variety of unusual compounds for survival. C-nucleosides isolated from Caribbean Cryptotethya crypta, were the basis for the synthesis of zidovudine (AZT), aciclovir (Cyclovir), cytarabine (Depocyt), and cytarabine derivative gemcitabine (Gemzar).
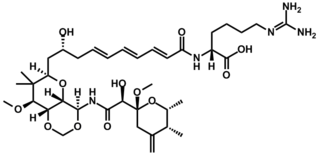
Onnamide A is a bioactive natural product found in Theonella swinhoei, a species of marine sponge whose genus is well known for yielding a diverse set of biologically active natural products, including the swinholides and polytheonamides. It bears structural similarities to the pederins, a family of compounds known to inhibit protein synthesis in eukaryotic cells. Onnamide A and its analogues have attracted academic interest due to their cytotoxicity and potential for combating the growth and proliferation of cancer cells.

Swinholides are dimeric 42 carbon-ring polyketides that exhibit a 2-fold axis of symmetry. Found mostly in the marine sponge Theonella, swinholides encompass cytotoxic and antifungal activities via disruption of the actin skeleton. Swinholides were first described in 1985 and the structure and stereochemistry were updated in 1989 and 1990, respectively. Thirteen swinholides have been described in the literature, including close structural compounds such as misakinolides/bistheonellides, ankaraholides, and hurgholide A It is suspected that symbiotic microbes that inhabit the sponges rather than the sponges themselves produce swinholides since the highest concentration of swinholides are found in the unicellular bacterial fraction of sponges and not in the sponge fraction or cyanobacteria fraction that also inhabit the sponges.
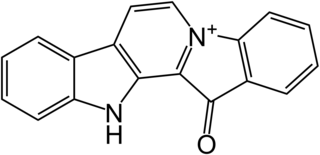
Fascaplysin is a marine alkaloid based on 12H-pyrido[1–2-a:3,4-b′]diindole ring system. It was first isolated as a red pigment from the marine sponge Fascaplysinopsis reticulata that was collected in the South Pacific near Fiji in 1988. Fascaplysin possesses a broad range of in vitro biological activities including analgesic, antimicrobial, antifungal, antiviral, antimalarial, anti-angiogenic, and antiproliferative activity against numerous cancer cell lines.

















