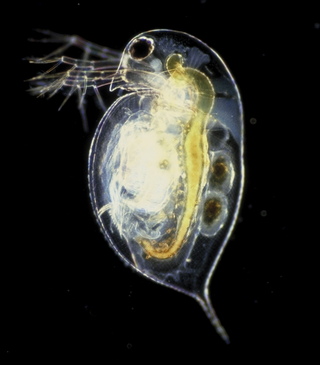
Daphnia is a genus of small planktonic crustaceans, 0.2–6.0 mm (0.01–0.24 in) in length. Daphnia are members of the order Anomopoda, and are one of the several small aquatic crustaceans commonly called water fleas because their saltatory swimming style resembles the movements of fleas. Daphnia spp. live in various aquatic environments ranging from acidic swamps to freshwater lakes and ponds.
Nosema apis is a microsporidian, a small, unicellular parasite recently reclassified as a fungus that mainly affects honey bees. It causes nosemosis, also called nosema, which is the most common and widespread of adult honey bee diseases. The dormant stage of N. apis is a long-lived spore which is resistant to temperature extremes and dehydration, and cannot be killed by freezing the contaminated comb. Nosemosis is a listed disease with the Office International des Epizooties (OIE).

Microsporidia are a group of spore-forming unicellular parasites. These spores contain an extrusion apparatus that has a coiled polar tube ending in an anchoring disc at the apical part of the spore. They were once considered protozoans or protists, but are now known to be fungi, or a sister group to fungi. These fungal microbes are obligate eukaryotic parasites that use a unique mechanism to infect host cells. They have recently been discovered in a 2017 Cornell study to infect Coleoptera on a large scale. So far, about 1500 of the probably more than one million species are named. Microsporidia are restricted to animal hosts, and all major groups of animals host microsporidia. Most infect insects, but they are also responsible for common diseases of crustaceans and fish. The named species of microsporidia usually infect one host species or a group of closely related taxa. Approximately 10 percent of the species are parasites of vertebrates —several species, most of which are opportunistic, can infect humans, in whom they can cause microsporidiosis.
Microsporidiosis is an opportunistic intestinal infection that causes diarrhea and wasting in immunocompromised individuals. It results from different species of microsporidia, a group of microbial (unicellular) fungi.

Thelytoky is a type of parthenogenesis and is the absence of mating and subsequent production of all female diploid offspring as for example in aphids. Thelytokous parthenogenesis is rare among animals and reported in about 1,500 species, about 1 in 1000 of described animal species, according to a 1984 study. It is more common in invertebrates, like arthropods, but it can occur in vertebrates, including salamanders, fish, and reptiles such as some whiptail lizards.

A xenoma is a growth caused by various protists and fungi, most notably microsporidia. It can occur on numerous organisms; however is predominantly found on fish.
Drosophila C virus (DCV) belongs to the genus Cripavirus and was previously thought to be a member of the virus family Picornaviridae; it has since been classified as belonging to the Dicistroviridae. It is a single stranded positive sense RNA virus of approximately 9300 nucleotides and it contains two open reading frames. The virus particles are 30 nm in diameter and are made up of approximately 30% of RNA and 70% protein. The virus capsid is composed of three major polypeptides and two minor polypeptides.
Encephalitozoon intestinalis is a parasite. It can cause microsporidiosis.

Encephalitozoon cuniculi is a microsporidial parasite of mammals with world-wide distribution. An important cause of neurologic and renal disease in rabbits, E. cuniculi can also cause disease in immunocompromised people.
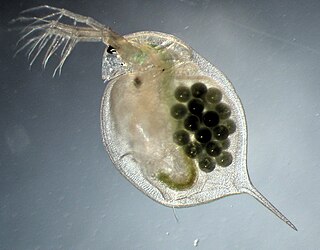
Daphnia magna is a small planktonic crustacean that belongs to the subclass Phyllopoda.
Host–parasite coevolution is a special case of coevolution, where a host and a parasite continually adapt to each other. This can create an evolutionary arms race between them. A more benign possibility is of an evolutionary trade-off between transmission and virulence in the parasite, as if it kills its host too quickly, the parasite will not be able to reproduce either. Another theory, the Red Queen hypothesis, proposes that since both host and parasite have to keep on evolving to keep up with each other, and since sexual reproduction continually creates new combinations of genes, parasitism favours sexual reproduction in the host.
Nosema bombi is a microsporidian, a small, unicellular parasite recently reclassified as a fungus that mainly affects bumble bees. It was reclassified as Vairimorpha bombi in 2020. The parasite infects numerous Bombus spp. at variable rates, and has been found to have a range of deleterious effects on its hosts.
Pasteuria is a genus of mycelial and endospore-forming, nonmotile gram-positive bacteria that are obligate parasites of some nematodes and crustaceans. The genus of Pasteuria was previously classified within the family Alicyclobacillaceae, but has since been moved to the family Pasteuriaceae.
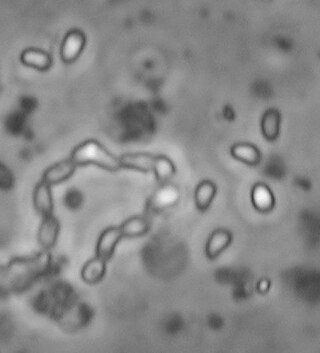
Ordospora colligata is an intracellular parasite belonging to the Microsporidia. It is an obligatory gut parasite with the crustacean Daphnia magna as its only host. So far it has been reported from Europe and Asia.

Pasteuria ramosa is a gram-positive, endospore-forming bacterium in the Bacillus/Clostridia clade within Bacillota. It is an obligate pathogen of cladoceran crustaceans from the genus Daphnia. Daphnia is a genus of small planktonic crustaceans including D. magna, P. ramosa's most popular host target. Other hosts include D. pulex, D. longispina, D. dentifera, and Moina rectirostris. An established and widely used coevolutionary model of host-pathogen interactions exists with P. ramosa and D. magna.
Nematocida parisii is a parasitic species of Microsporidia fungi found in wild isolates of the common nematode, Caenorhabditis elegans. The fungus forms spores and replicates in the intestines before leaving the host.

Dieter Ebert is professor for Zoology and Evolutionary Biology at the Zoological Institute at the University of Basel in Basel, Switzerland. He is an evolutionary ecologist and geneticist, known for his research on host–pathogen interaction and coevolution, mainly using the model system Daphnia and its parasites.

Enterospora nucleophila is a microsporidian infecting the gilt-head bream. It develops primarily within the nuclei of rodlet cells and enterocytes, at the intestinal epithelium. It can also be found in cytoplasmic position within other cell types, including phagocytes, at subepithelial layers. It is the causative agent of emaciative microsporidiosis of gilthead sea bream, a chronic condition manifested as a severe growth arrestment, normally accompanied by trickling mortality.
Hazardia is a genus of microsporidians that parasite insects, with the type host being Culex pipiens. It is currently classified as incertae sedis within the order Amblyosporida of phylum Rozellomycota.












