Related Research Articles

Proteobacteria is a major phylum of Gram-negative bacteria. They include a wide variety of pathogenic genera, such as Escherichia, Salmonella, Vibrio, Helicobacter, Yersinia, Legionellales, and many others. Others are free-living (nonparasitic) and include many of the bacteria responsible for nitrogen fixation.

Redox is a type of chemical reaction in which the oxidation states of atoms are changed. Redox reactions are characterized by the actual or formal transfer of electrons between chemical species, most often with one species undergoing oxidation while another species undergoes reduction. The chemical species from which the electron is removed is said to have been oxidized, while the chemical species to which the electron is added is said to have been reduced. In other words:

Iron ores are rocks and minerals from which metallic iron can be economically extracted. The ores are usually rich in iron oxides and vary in color from dark grey, bright yellow, or deep purple to rusty red. The iron is usually found in the form of magnetite (Fe
3O
4, 72.4% Fe), hematite (Fe
2O
3, 69.9% Fe), goethite (FeO(OH), 62.9% Fe), limonite (FeO(OH)·n(H2O), 55% Fe) or siderite (FeCO3, 48.2% Fe).
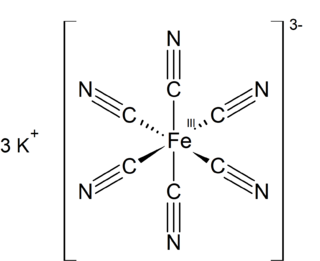
Potassium ferricyanide is the chemical compound with the formula K3[Fe(CN)6]. This bright red salt contains the octahedrally coordinated [Fe(CN)6]3− ion. It is soluble in water and its solution shows some green-yellow fluorescence. It was discovered in 1822 by Leopold Gmelin, and was initially used in the production of ultramarine dyes.
A reducing agent is an element or compound that loses an electron to an electron recipient in a redox chemical reaction.

Iron oxides are chemical compounds composed of iron and oxygen. There are sixteen known iron oxides and oxyhydroxides, the best known of which is rust, a form of iron(III) oxide.
In biochemistry, chemosynthesis is the biological conversion of one or more carbon-containing molecules and nutrients into organic matter using the oxidation of inorganic compounds or ferrous ions as a source of energy, rather than sunlight, as in photosynthesis. Chemoautotrophs, organisms that obtain carbon from carbon dioxide through chemosynthesis, are phylogenetically diverse, but also groups that include conspicuous or biogeochemically-important taxa include the sulfur-oxidizing gamma and epsilon proteobacteria, the Aquificae, the methanogenic archaea and the neutrophilic iron-oxidizing bacteria.
Anaerobic respiration is respiration using electron acceptors other than molecular oxygen (O2). Although oxygen is not the final electron acceptor, the process still uses a respiratory electron transport chain.
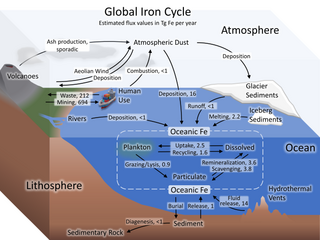
The iron cycle (Fe) is the biogeochemical cycle of iron through the atmosphere, hydrosphere, biosphere and lithosphere. While Fe is highly abundant in the Earth's crust, it is less common in oxygenated surface waters. Iron is a key micronutrient in primary productivity, and a limiting nutrient in the Southern ocean, eastern equatorial Pacific, and the subarctic Pacific referred to as High-Nutrient, Low-Chlorophyll (HNLC) regions of the ocean.

Chemotrophs are organisms that obtain energy by the oxidation of electron donors in their environments. These molecules can be organic (chemoorganotrophs) or inorganic (chemolithotrophs). The chemotroph designation is in contrast to phototrophs, which use solar energy. Chemotrophs can be either autotrophic or heterotrophic. Chemotrophs are found in ocean floors where sunlight cannot reach them because of the great depth and all the levels of water inbetween them and the sun. They evolved to not be dependent on solar energy. Ocean floors often contain underwater volcanos that can provide heat as a substitute for sunlight's warmth.
NADPH oxidase is a membrane-bound enzyme complex that faces the extracellular space. It can be found in the plasma membrane as well as in the membranes of phagosomes used by neutrophil white blood cells to engulf microorganisms. Human isoforms of the catalytic component of the complex include NOX1, NOX2, NOX3, NOX4, NOX5, DUOX1, and DUOX2.

Iron-oxidizing bacteria are chemotrophic bacteria that derive the energy they need to live and multiply by oxidizing dissolved ferrous iron. They are known to grow and proliferate in waters containing iron concentrations as low as 0.1 mg/L. However, at least 0.3 ppm of dissolved oxygen is needed to carry out oxidation.
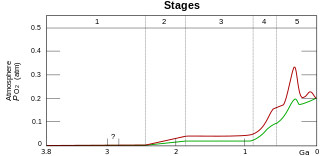
The Great Oxidation Event (GOE), sometimes also called the Great Oxygenation Event, Oxygen Catastrophe, Oxygen Crisis, Oxygen Holocaust, or Oxygen Revolution, was a time period when the Earth's atmosphere and the shallow ocean first experienced a rise in oxygen, approximately 2.4 - 2.0 Ga (billion years ago) during the Paleoproterozoic era. Geological, isotopic, and chemical evidence suggest that biologically produced molecular oxygen (dioxygen, O2) started to accumulate in Earth's atmosphere and changed it from a weakly reducing atmosphere to an oxidizing atmosphere, causing many existing species on Earth to die out. The cyanobacteria producing the oxygen caused the event which enabled the subsequent development of multicellular forms.
Microbial metabolism is the means by which a microbe obtains the energy and nutrients it needs to live and reproduce. Microbes use many different types of metabolic strategies and species can often be differentiated from each other based on metabolic characteristics. The specific metabolic properties of a microbe are the major factors in determining that microbe's ecological niche, and often allow for that microbe to be useful in industrial processes or responsible for biogeochemical cycles.

The class Zetaproteobacteria is the sixth and most recently described class of the Proteobacteria. Zetaproteobacteria can also refer to the group of organisms assigned to this class. The Zetaproteobacteria were originally represented by a single described species, Mariprofundus ferrooxydans, which is an iron-oxidizing neutrophilic chemolithoautotroph originally isolated from Loihi Seamount in 1996 (post-eruption). Molecular cloning techniques focusing on the small subunit ribosomal RNA gene have also been used to identify a more diverse majority of the Zetaproteobacteria that have as yet been unculturable.

Mariprofundus ferrooxydans is a neutrophilic, chemolithotrophic, Gram-negative bacterium which can grow by oxidising ferrous to ferric iron. It is one of the few members of the class Zetaproteobacteria in the phylum Proteobacteria.
Advenella kashmirensis is a chemolithotrophic, mesophilic, neutrophilic, tetrathionate-oxidizing bacterium of the genus Advenella, isolated from the soil of a temperate orchard in Jammu and Kashmir in India. Tetrathiobacter kashmirensis has been reclassified to Advenella kashmirensis. The complete genome of A. kashmirensis has been sequenced.
Hoeflea is a genus of Gram-negative, strictly aerobic, oxidase- and catalase-positive, non-spore-forming, rod-shaped bacteria.
Hoeflea halophila is a Gram-negative, aerobic, motile bacteria from the genus of Hoeflea which was isolated from marine sediment from the Sea of Japan.
Dissimilatory metal-reducing microorganisms are a group of microorganisms (both bacteria and archaea) that can perform anaerobic respiration utilizing a metal as terminal electron acceptor rather than molecular oxygen (O2), which is the terminal electron acceptor reduced to water (H2O) in aerobic respiration. The most common metals used for this end are iron [Fe(III)] and manganese [Mn(IV)], which are reduced to Fe(II) and Mn(II) respectively, and most microorganisms that reduce Fe(III) can reduce Mn(IV) as well. But other metals and metalloids are also used as terminal electron acceptors, such as vanadium [V(V)], chromium [Cr(VI)], molybdenum [Mo(VI)], cobalt [Co(III)], palladium [Pd(II)], gold [Au(III)], and mercury [Hg(II)].
References
| This Phyllobacteriaceae article is a stub. You can help Wikipedia by expanding it. |