Related Research Articles
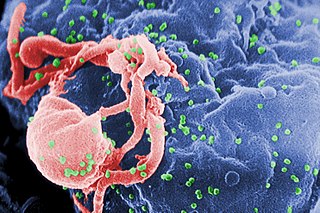
The human immunodeficiency viruses (HIV) are two species of Lentivirus that infect humans. Over time, they cause acquired immunodeficiency syndrome (AIDS), a condition in which progressive failure of the immune system allows life-threatening opportunistic infections and cancers to thrive. Without treatment, the average survival time after infection with HIV is estimated to be 9 to 11 years, depending on the HIV subtype.

Inflammation is part of the biological response of body tissues to harmful stimuli, such as pathogens, damaged cells, or irritants. The five cardinal signs are heat, pain, redness, swelling, and loss of function.

Macrophages are a type of white blood cell of the innate immune system that engulf and digest pathogens, such as cancer cells, microbes, cellular debris, and foreign substances, which do not have proteins that are specific to healthy body cells on their surface. This process is called phagocytosis, which acts to defend the host against infection and injury.

Kupffer cells, also known as stellate macrophages and Kupffer–Browicz cells, are specialized cells localized in the liver within the lumen of the liver sinusoids and are adhesive to their endothelial cells which make up the blood vessel walls. Kupffer cells comprise the largest population of tissue-resident macrophages in the body. Gut bacteria, bacterial endotoxins, and microbial debris transported to the liver from the gastrointestinal tract via the portal vein will first come in contact with Kupffer cells, the first immune cells in the liver. It is because of this that any change to Kupffer cell functions can be connected to various liver diseases such as alcoholic liver disease, viral hepatitis, intrahepatic cholestasis, steatohepatitis, activation or rejection of the liver during liver transplantation and liver fibrosis. They form part of the mononuclear phagocyte system.

Microglia are a type of neuroglia located throughout the brain and spinal cord. Microglia account for about 10-15% of cells found within the brain. As the resident macrophage cells, they act as the first and main form of active immune defense in the central nervous system (CNS). Microglia originate in the yolk sac under a tightly regulated molecular process. These cells are distributed in large non-overlapping regions throughout the CNS. Microglia are key cells in overall brain maintenance—they are constantly scavenging the CNS for plaques, damaged or unnecessary neurons and synapses, and infectious agents. Since these processes must be efficient to prevent potentially fatal damage, microglia are extremely sensitive to even small pathological changes in the CNS. This sensitivity is achieved in part by the presence of unique potassium channels that respond to even small changes in extracellular potassium. Recent evidence shows that microglia are also key players in the sustainment of normal brain functions under healthy conditions. Microglia also constantly monitor neuronal functions through direct somatic contacts and exert neuroprotective effects when needed.
Following infection with HIV-1, the rate of clinical disease progression varies between individuals. Factors such as host susceptibility, genetics and immune function, health care and co-infections as well as viral genetic variability may affect the rate of progression to the point of needing to take medication in order not to develop AIDS.
HIV-associated neurocognitive disorders (HAND) are neurological disorders associated with HIV infection and AIDS. It is a syndrome of progressive deterioration of memory, cognition, behavior, and motor function in HIV-infected individuals during the late stages of the disease, when immunodeficiency is severe. HAND may include neurological disorders of various severity. HIV-associated neurocognitive disorders are associated with a metabolic encephalopathy induced by HIV infection and fueled by immune activation of macrophages and microglia. These cells are actively infected with HIV and secrete neurotoxins of both host and viral origin. The essential features of HIV-associated dementia (HAD) are disabling cognitive impairment accompanied by motor dysfunction, speech problems and behavioral change. Cognitive impairment is characterised by mental slowness, trouble with memory and poor concentration. Motor symptoms include a loss of fine motor control leading to clumsiness, poor balance and tremors. Behavioral changes may include apathy, lethargy and diminished emotional responses and spontaneity. Histopathologically, it is identified by the infiltration of monocytes and macrophages into the central nervous system (CNS), gliosis, pallor of myelin sheaths, abnormalities of dendritic processes and neuronal loss.
Neuroimmunology is a field combining neuroscience, the study of the nervous system, and immunology, the study of the immune system. Neuroimmunologists seek to better understand the interactions of these two complex systems during development, homeostasis, and response to injuries. A long-term goal of this rapidly developing research area is to further develop our understanding of the pathology of certain neurological diseases, some of which have no clear etiology. In doing so, neuroimmunology contributes to development of new pharmacological treatments for several neurological conditions. Many types of interactions involve both the nervous and immune systems including the physiological functioning of the two systems in health and disease, malfunction of either and or both systems that leads to disorders, and the physical, chemical, and environmental stressors that affect the two systems on a daily basis.

The neuroimmune system is a system of structures and processes involving the biochemical and electrophysiological interactions between the nervous system and immune system which protect neurons from pathogens. It serves to protect neurons against disease by maintaining selectively permeable barriers, mediating neuroinflammation and wound healing in damaged neurons, and mobilizing host defenses against pathogens.

CX3C motif chemokine receptor 1 (CX3CR1), also known as the fractalkine receptor or G-protein coupled receptor 13 (GPR13), is a transmembrane protein of the G protein-coupled receptor 1 (GPCR1) family and the only known member of the CX3C chemokine receptor subfamily.

Epithelioid cells are derivatives of activated macrophages resembling epithelial cells.
The central nervous system (CNS) controls most of the functions of the body and mind. It comprises the brain, spinal cord and the nerve fibers that branch off to all parts of the body. The CNS viral diseases are caused by viruses that attack the CNS. Existing and emerging viral CNS infections are major sources of human morbidity and mortality.

Leishmania donovani is a species of intracellular parasites belonging to the genus Leishmania, a group of haemoflagellate kinetoplastids that cause the disease leishmaniasis. It is a human blood parasite responsible for visceral leishmaniasis or kala-azar, the most severe form of leishmaniasis. It infects the mononuclear phagocyte system including spleen, liver and bone marrow. Infection is transmitted by species of sandfly belonging to the genus Phlebotomus in Old World and Lutzomyia in New World. The species complex it represents is prevalent throughout tropical and temperate regions including Africa, China, India, Nepal, southern Europe, Russia and South America. The species complex is responsible for thousands of deaths every year and has spread to 88 countries, with 350 million people at constant risk of infection and 0.5 million new cases in a year.

Janice Ellen Clements is vice dean for faculty at the Johns Hopkins School of Medicine and the Mary Wallace Stanton Professor of Faculty Affairs. She is a professor in the departments of Molecular and Comparative Pathobiology, Neurology, and Pathology, and has a joint appointment in molecular biology and genetics. Her molecular biology and virology research examines lentiviruses and how they cause neurological diseases.

HIV/AIDS research includes all medical research that attempts to prevent, treat, or cure HIV/AIDS, as well as fundamental research about the nature of HIV as an infectious agent and AIDS as the disease caused by HIV.
Kalipada Pahan is a professor of Neurological Sciences, Biochemistry and Pharmacology, and the Floyd A. Davis, M.D., Endowed Chair in Neurology at the Rush University Medical Center. He is also a research career scientist at the Department of Veterans Affairs, Jesse Brown VA Medical Center. He is an eminent Indian American neuroscientist involved in translational research on multiple sclerosis, Parkinson's disease, Alzheimer's disease, dementia, and Batten disease. He is well known for his research on statins, cholesterol-lowering drugs. He first explored the application of statins in suppressing the inflammatory events in microglia, astroglia and macrophages. This finding has revolutionized the research on statin drugs. Later, his lab has shown that statins may be beneficial in protecting neurons and improving locomotor activities in Parkinson's disease by suppressing the activation of p21/Ras. His lab is also famous for research on cinnamon where they have described that this commonly-used natural spice may be beneficial for different brain disorders including improving memory and learning of poor learners. Recently his lab has delineated a unique crosstalk between fat and memory in which the lipid-lowering transcription factor PPARalpha controls the formation of hippocampal memory via transcriptional regulation of CREB, suggesting a possible reason for the connection between excess belly fat and memory loss.
Neuroinflammation is inflammation of the nervous tissue. It may be initiated in response to a variety of cues, including infection, traumatic brain injury, toxic metabolites, or autoimmunity. In the central nervous system (CNS), including the brain and spinal cord, microglia are the resident innate immune cells that are activated in response to these cues. The CNS is typically an immunologically privileged site because peripheral immune cells are generally blocked by the blood–brain barrier (BBB), a specialized structure composed of astrocytes and endothelial cells. However, circulating peripheral immune cells may surpass a compromised BBB and encounter neurons and glial cells expressing major histocompatibility complex molecules, perpetuating the immune response. Although the response is initiated to protect the central nervous system from the infectious agent, the effect may be toxic and widespread inflammation as well as further migration of leukocytes through the blood–brain barrier may occur.

Sharon Ruth Lewin, FRACP, FAHMS is a leading infectious diseases expert who is the inaugural Director of The Peter Doherty Institute for Infection and Immunity and the Cumming Global Centre for Pandemic Therapeutics. She is also a Melbourne Laureate Professor of Medicine at The University of Melbourne, and the current President of the International AIDS Society (IAS).
Georgette D. Kanmogne is a Cameroonian American geneticist and molecular virologist and a full professor and vice chair for resource allocation and faculty development within the Department of Pharmacology and Experimental Neurosciences at the University of Nebraska Medical Center in Omaha, Nebraska. Kanmogne's research program focuses on exploring the pathogenesis of neuroAIDS by deciphering the mechanisms underlying blood brain barrier dysfunction and viral entry into the central nervous system. Her research also addresses the lack of HIV therapies that cross the blood brain barrier (BBB) and has played a critical role in the development of nanoparticles encapsulating HIV-drugs that can cross the BBB to prevent viral-mediated neuron death in the brain. Kanmogne collaborates with clinical and basic researchers across America, Cameroon, and West Africa, spanning disciplines from hematology to psychiatry, to explore how viral genetic diversity is correlated with the neurological impact of HIV.

Amanda Brown is an American immunologist and microbiologist as well as an associate professor of neurology and neuroscience at Johns Hopkins University School of Medicine in Baltimore, Maryland. Brown is notable for cloning one of the first recombinant HIV viruses and developing a novel method to visualize HIV infected cells using GFP fluorescence.
References
- ↑ Johnson, Amanda McGill (2022-01-28). "2022 Luncheon Honoreers". Nebraska Cures. Retrieved 2022-10-26./
- ↑ Temple University Health System (2019-07-02). "HIV eliminated from the genomes of living animals". Science Daily=en-US. Retrieved 2022-10-26.
- 1 2 3 4 Utesch, Margie (2018-07-03). "Humanitarian of the Year". The Jewish Community Center of Omaha. Retrieved 2021-12-16.
- ↑ "Potential new HIV treatment developed at UNMC". KMTV. 2020-04-29. Retrieved 2021-12-16.
- ↑ "Front Line (2009)" (PDF). University of Nebraska Medical Center, Department of Pharmacology and Experimental Neuroscience. Retrieved 2022-01-25.
- ↑ "About Us". University of Nebraska Medical Center, Department of Pharmacology and Experimental Neuroscience. Retrieved 2022-01-25.
- ↑ "UNMC Professor gets Fulbright to study in Israel (2001)". Daily Nebraskan. 21 March 2000. Retrieved 2022-11-04.
- 1 2 "The Pioneer in Neurovirology Award". International Society for NeurVirology. 2016. Retrieved 4 November 2022.
- ↑ Adachi, A; Gendelman, H E; Koenig, S; Folks, T; Willey, R; Rabson, A; Martin, M A (August 1986). "Production of acquired immunodeficiency syndrome-associated retrovirus in human and nonhuman cells transfected with an infectious molecular clone". Journal of Virology. 59 (2): 284–291. doi:10.1128/jvi.59.2.284-291.1986. ISSN 0022-538X. PMC 253077 . PMID 3016298. S2CID 12551511.
- ↑ Koenig, Scott; Gendelman, Howard E.; Orenstein, Jan M.; Dal Canto, Mauro C.; Pezeshkpour, Gholam H.; Yungbluth, Margaret; Janotta, Frank; Aksamit, Allen; Martin, Malcolm A.; Fauci, Anthony S. (1986-09-05). "Detection of AIDS Virus in Macrophages in Brain Tissue from AIDS Patients with Encephalopathy". Science. 233 (4768): 1089–1093. Bibcode:1986Sci...233.1089K. doi:10.1126/science.3016903. ISSN 0036-8075. PMID 3016903.
- ↑ Gendelman, H. E.; Narayan, O.; Molineaux, S.; Clements, J. E.; Ghotbi, Z. (October 1985). "Slow, persistent replication of lentiviruses: role of tissue macrophages and macrophage precursors in bone marrow". Proceedings of the National Academy of Sciences of the United States of America. 82 (20): 7086–7090. Bibcode:1985PNAS...82.7086G. doi: 10.1073/pnas.82.20.7086 . ISSN 0027-8424. PMC 391315 . PMID 2996004.
- ↑ Gendelman, Howard E.; Zheng, Jialin; Coulter, Cynthia L.; Ghorpade, Anuja; Che, Myhanh; Thylin, Michael; Rubocki, Ronald; Persidsky, Yuri; Hahn, Francis; Reinhard, Jr., John; Swindells, Susan (October 1998). "Suppression of Inflammatory Neurotoxins by Highly Active Antiretroviral Therapy in Human Immunodeficiency Virus-Associated Dementia". The Journal of Infectious Diseases. 178 (4): 1000–1007. doi: 10.1086/515693 . ISSN 0022-1899. PMID 9806027. S2CID 42427863.
- ↑ Spellman, Lisa (2019-08-09). "Science Cafe explores possibility of HIV cure". University of Nebraska Medical Center. Retrieved 2021-12-16.
- ↑ Gendelman, Howard E. (2012). The neurology of AIDS. Oxford University Press. ISBN 978-0-19-539934-9. OCLC 828615707.
- ↑ "Scientists say they found a cure for HIV in some mice. Humans could be next". www.washingtonpost.com. Retrieved 2022-11-23.
- ↑ "In a first, scientists eliminate HIV from an animal's genome". www.cbsnews.com. 3 July 2019. Retrieved 2021-12-17.
- ↑ Yancey-Bragg, N'dea. "Researchers have eliminated HIV in mice for the first time. Is a cure for humans next?". USA TODAY. Retrieved 2021-12-17.
- ↑ Dash, Prasanta K.; Kaminski, Rafal; Bella, Ramona; Su, Hang; Mathews, Saumi; Ahooyi, Taha M.; Chen, Chen; Mancuso, Pietro; Sariyer, Rahsan; Ferrante, Pasquale; Donadoni, Martina (December 2019). "Sequential LASER ART and CRISPR Treatments Eliminate HIV-1 in a Subset of Infected Humanized Mice". Nature Communications. 10 (1): 2753. Bibcode:2019NatCo..10.2753D. doi:10.1038/s41467-019-10366-y. ISSN 2041-1723. PMC 6606613 . PMID 31266936.
- ↑ Kulkarni, Tanmay A.; Bade, Aditya N.; Sillman, Brady; Shetty, Bhagya Laxmi Dyavar; Wojtkiewicz, Melinda S.; Gautam, Nagsen; Hilaire, James R.; Sravanam, Sruthi; Szlachetka, Adam; Lamberty, Benjamin G.; Morsey, Brenda M. (August 2020). "A year-long extended release nanoformulated cabotegravir prodrug". Nature Materials. 19 (8): 910–920. Bibcode:2020NatMa..19..910K. doi:10.1038/s41563-020-0674-z. ISSN 1476-1122. PMC 7384935 . PMID 32341511.
- ↑ Soriano, Vicente; Barreiro, Pablo; de Mendoza, Carmen (August 2020). "Long-acting antiretroviral therapy". Nature Materials. 19 (8): 826–827. Bibcode:2020NatMa..19..826S. doi: 10.1038/s41563-020-0731-7 . ISSN 1476-4660. PMID 32704135. S2CID 220721631.
- ↑ "Nebraska Nanomedicine Production Plant | Pharmacology | University of Nebraska Medical Center". www.unmc.edu. Retrieved 2021-12-16.
- ↑ "Exavir Therapeutics". exavirtherapeutics.com. 2021-02-14. Retrieved 2021-12-16.[ permanent dead link ]
- ↑ Benner, Eric J.; Mosley, R. Lee; Destache, Chris J.; Lewis, Travis B.; Jackson-Lewis, Vernice; Gorantla, Santhi; Nemachek, Craig; Green, Steven R.; Przedborski, Serge; Gendelman, Howard E. (2004-06-22). "Therapeutic immunization protects dopaminergic neurons in a mouse model of Parkinson's disease". Proceedings of the National Academy of Sciences of the United States of America. 101 (25): 9435–9440. Bibcode:2004PNAS..101.9435B. doi: 10.1073/pnas.0400569101 . ISSN 0027-8424. PMC 438994 . PMID 15197276.
- ↑ Gendelman, Howard E.; Zhang, Yuning; Santamaria, Pamela; Olson, Katherine E.; Schutt, Charles R.; Bhatti, Danish; Shetty, Bhagya Laxmi Dyavar; Lu, Yaman; Estes, Katherine A.; Standaert, David G.; Heinrichs-Graham, Elizabeth (2017-03-23). "Evaluation of the safety and immunomodulatory effects of sargramostim in a randomized, double-blind phase 1 clinical Parkinson's disease trial". npj Parkinson's Disease. 3 (1): 10. doi:10.1038/s41531-017-0013-5. ISSN 2373-8057. PMC 5445595 . PMID 28649610.
- ↑ Olson, Katherine E.; Namminga, Krista L.; Lu, Yaman; Schwab, Aaron D.; Thurston, Mackenzie J.; Abdelmoaty, Mai M.; Kumar, Vikas; Wojtkiewicz, Melinda; Obaro, Helen; Santamaria, Pamela; Mosley, R. Lee (2021-05-01). "Safety, tolerability, and immune-biomarker profiling for year-long sargramostim treatment of Parkinson's disease". eBioMedicine. 67: 103380. doi:10.1016/j.ebiom.2021.103380. ISSN 2352-3964. PMC 8138485 . PMID 34000620.
- ↑ Gendelman, Howard E; Grant, Igor; Everall, Ian Paul; Fox, Howard S; Gelbard, Harris A; Lipton, Stuart A; Swindells, Susan, eds. (2012). The Neurology of AIDS. Oxford University Press. doi:10.1093/med/9780195399349.001.0001. ISBN 978-0-19-996519-9.
- ↑ Ikezu, Tsuneya; Gendelman, Howard E., eds. (2017). Neuroimmune Pharmacology. doi:10.1007/978-3-319-44022-4. ISBN 978-3-319-44020-0.
- ↑ "Innovation Awards". UNeMed.com. 23 October 2022. Retrieved 23 November 2022.
- ↑ "2022 Luncheon Honorees". Nebraska Cures. 28 January 2022. Retrieved 28 April 2022.
- ↑ "Tribute to Dr. Howard Gendelman and Dr. Bonnie Bloch". YouTube. Nebraska Cures. Retrieved 28 April 2022.
- ↑ "SNIP - Society on NeuroImmune Pharmacology". s-nip.org. Retrieved 2021-12-16.
- ↑ "$3 Million Grant Recognizes Potential of Research at the UNMC Center for Neurovirology and Neurodegenerative Disorders". University of Nebraska Medical Center. 2001. Retrieved 4 November 2022.