Related Research Articles
Bioleaching is the extraction or liberation of metals from their ores through the use of living organisms. Bioleaching is one of several applications within biohydrometallurgy and several methods are used to treat ores or concentrates containing copper, zinc, lead, arsenic, antimony, nickel, molybdenum, gold, silver, and cobalt.
Extractive metallurgy is a branch of metallurgical engineering wherein process and methods of extraction of metals from their natural mineral deposits are studied. The field is a materials science, covering all aspects of the types of ore, washing, concentration, separation, chemical processes and extraction of pure metal and their alloying to suit various applications, sometimes for direct use as a finished product, but more often in a form that requires further working to achieve the given properties to suit the applications.

Chalcopyrite ( KAL-kə-PY-ryte, -koh-) is a copper iron sulfide mineral and the most abundant copper ore mineral. It has the chemical formula CuFeS2 and crystallizes in the tetragonal system. It has a brassy to golden yellow color and a hardness of 3.5 to 4 on the Mohs scale. Its streak is diagnostic as green-tinged black.
Hydrothermal circulation in its most general sense is the circulation of hot water. Hydrothermal circulation occurs most often in the vicinity of sources of heat within the Earth's crust. In general, this occurs near volcanic activity, but can occur in the shallow to mid crust along deeply penetrating fault irregularities or in the deep crust related to the intrusion of granite, or as the result of orogeny or metamorphism. Hydrothermal circulation often results in hydrothermal mineral deposits.

Bornite, also known as peacock ore, is a sulfide mineral with chemical composition Cu5FeS4 that crystallizes in the orthorhombic system (pseudo-cubic).

Skarns or tactites are coarse-grained metamorphic rocks that form by replacement of carbonate-bearing rocks during regional or contact metamorphism and metasomatism. Skarns may form by metamorphic recrystallization of impure carbonate protoliths, bimetasomatic reaction of different lithologies, and infiltration metasomatism by magmatic-hydrothermal fluids. Skarns tend to be rich in calcium-magnesium-iron-manganese-aluminium silicate minerals, which are also referred to as calc-silicate minerals. These minerals form as a result of alteration which occurs when hydrothermal fluids interact with a protolith of either igneous or sedimentary origin. In many cases, skarns are associated with the intrusion of a granitic pluton found in and around faults or shear zones that commonly intrude into a carbonate layer composed of either dolomite or limestone. Skarns can form by regional or contact metamorphism and therefore form in relatively high temperature environments. The hydrothermal fluids associated with the metasomatic processes can originate from a variety of sources; magmatic, metamorphic, meteoric, marine, or even a mix of these. The resulting skarn may consist of a variety of different minerals which are highly dependent on both the original composition of the hydrothermal fluid and the original composition of the protolith.

Copper extraction refers to the methods used to obtain copper from its ores. The conversion of copper ores consists of a series of physical, chemical and electrochemical processes. Methods have evolved and vary with country depending on the ore source, local environmental regulations, and other factors.

Volcanogenic massive sulfide ore deposits, also known as VMS ore deposits, are a type of metal sulfide ore deposit, mainly copper-zinc which are associated with and produced by volcanic-associated hydrothermal events in submarine environments.

Porphyry copper deposits are copper ore bodies that are formed from hydrothermal fluids that originate from a voluminous magma chamber several kilometers below the deposit itself. Predating or associated with those fluids are vertical dikes of porphyritic intrusive rocks from which this deposit type derives its name. In later stages, circulating meteoric fluids may interact with the magmatic fluids. Successive envelopes of hydrothermal alteration typically enclose a core of disseminated ore minerals in often stockwork-forming hairline fractures and veins. Because of their large volume, porphyry orebodies can be economic from copper concentrations as low as 0.15% copper and can have economic amounts of by-products such as molybdenum, silver, and gold. In some mines, those metals are the main product.

Various theories of ore genesis explain how the various types of mineral deposits form within Earth's crust. Ore-genesis theories vary depending on the mineral or commodity examined.

Sedimentary exhalative deposits are zinc-lead deposits originally interpreted to have been formed by discharge of metal-bearing basinal fluids onto the seafloor resulting in the precipitation of mainly stratiform ore, often with thin laminations of sulfide minerals. SEDEX deposits are hosted largely by clastic rocks deposited in intracontinental rifts or failed rift basins and passive continental margins. Since these ore deposits frequently form massive sulfide lenses, they are also named sediment-hosted massive sulfide (SHMS) deposits, as opposed to volcanic-hosted massive sulfide (VHMS) deposits. The sedimentary appearance of the thin laminations led to early interpretations that the deposits formed exclusively or mainly by exhalative processes onto the seafloor, hence the term SEDEX. However, recent study of numerous deposits indicates that shallow subsurface replacement is also an important process, in several deposits the predominant one, with only local if any exhalations onto the seafloor. For this reason, some authors prefer the term clastic-dominated zinc-lead deposits. As used today, therefore, the term SEDEX is not to be taken to mean that hydrothermal fluids actually vented into the overlying water column, although this may have occurred in some cases.
In ore deposit geology, supergene processes or enrichment are those that occur relatively near the surface as opposed to deep hypogene processes. Supergene processes include the predominance of meteoric water circulation (i.e. water derived from precipitation) with concomitant oxidation and chemical weathering. The descending meteoric waters oxidize the primary (hypogene) sulfide ore minerals and redistribute the metallic ore elements. Supergene enrichment occurs at the base of the oxidized portion of an ore deposit. Metals that have been leached from the oxidized ore are carried downward by percolating groundwater, and react with hypogene sulfides at the supergene-hypogene boundary. The reaction produces secondary sulfides with metal contents higher than those of the primary ore. This is particularly noted in copper ore deposits where the copper sulfide minerals chalcocite (Cu2S), covellite (CuS), digenite (Cu18S10), and djurleite (Cu31S16) are deposited by the descending surface waters.
Violarite (Fe2+Ni23+S4) is a supergene sulfide mineral associated with the weathering and oxidation of primary pentlandite nickel sulfide ore minerals.
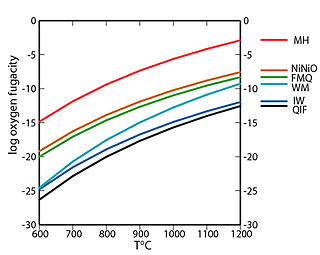
In geology, a redox buffer is an assemblage of minerals or compounds that constrains oxygen fugacity as a function of temperature. Knowledge of the redox conditions (or equivalently, oxygen fugacities) at which a rock forms and evolves can be important for interpreting the rock history. Iron, sulfur, and manganese are three of the relatively abundant elements in the Earth's crust that occur in more than one oxidation state. For instance, iron, the fourth most abundant element in the crust, exists as native iron, ferrous iron (Fe2+), and ferric iron (Fe3+). The redox state of a rock affects the relative proportions of the oxidation states of these elements and hence may determine both the minerals present and their compositions. If a rock contains pure minerals that constitute a redox buffer, then the oxygen fugacity of equilibration is defined by one of the curves in the accompanying fugacity-temperature diagram.

A polymetallic replacement deposit, also known as carbonate replacement deposit or high-temperature carbonate-hosted Ag-Pb-Zn deposit, is an orebody of metallic minerals formed by the replacement of sedimentary, usually carbonate rock, by metal-bearing solutions in the vicinity of igneous intrusions. When the ore forms a blanketlike body along the bedding plane of the rock, it is commonly called a manto ore deposit. Other ore geometries are chimneys and veins. Polymetallic replacements/mantos are often stratiform wall-rock replacement orebodies distal to porphyry copper deposits, or porphyry molybdenum deposits. The term manto is derived from the Spanish word manto, meaning "mantle" or "cloak".

Carbonate-hosted lead-zinc ore deposits are important and highly valuable concentrations of lead and zinc sulfide ores hosted within carbonate formations and which share a common genetic origin.
Partial melting is the phenomenon that occurs when a rock is subjected to temperatures high enough to cause certain minerals to melt, but not all of them. Partial melting is an important part of the formation of all igneous rocks and some metamorphic rocks, as evidenced by a multitude of geochemical, geophysical and petrological studies.

Cobalt extraction refers to the techniques used to extract cobalt from its ores and other compound ores. Several methods exist for the separation of cobalt from copper and nickel. They depend on the concentration of cobalt and the exact composition of the ore used.

A primary mineral is any mineral formed during the original crystallization of the host igneous primary rock and includes the essential mineral(s) used to classify the rock along with any accessory minerals. In ore deposit geology, hypogene processes occur deep below the Earth's surface, and tend to form deposits of primary minerals, as opposed to supergene processes that occur at or near the surface, and tend to form secondary minerals.
Hydrothermal mineral deposits are accumulations of valuable minerals which formed from hot waters circulating in Earth's crust through fractures. They eventually produce metallic-rich fluids concentrated in a selected volume of rock, which become supersaturated and then precipitate ore minerals. In some occurrences, minerals can be extracted for a profit by mining. Discovery of mineral deposits consumes considerable time and resources and only about one in every one thousand prospects explored by companies are eventually developed into a mine. A mineral deposit is any geologically significant concentration of an economically useful rock or mineral present in a specified area. The presence of a known but unexploited mineral deposit implies a lack of evidence for profitable extraction.
References
- ↑ Rakovan, John (November–December 2003). "A Word to the Wise: Hypogene & Supergene" (PDF). Rocks & Minerals. 78 (6). Taylor & Francis: 419. Bibcode:2003RoMin..78..419R. doi:10.1080/00357529.2003.9926759. S2CID 128609800 . Retrieved August 18, 2012.
- 1 2 The Encyclopedia of Gemstones and Minerals (1991). Martin Holden. Publisher: Facts on File
- 1 2 Understanding Mineral Deposits (2000). Kula C Misra. Kluwer Academic Publishers