Bioleaching is the extraction or liberation of metals from their ores through the use of living organisms. Bioleaching is one of several applications within biohydrometallurgy and several methods are used to treat ores or concentrates containing copper, zinc, lead, arsenic, antimony, nickel, molybdenum, gold, silver, and cobalt.
Extractive metallurgy is a branch of metallurgical engineering wherein process and methods of extraction of metals from their natural mineral deposits are studied. The field is a materials science, covering all aspects of the types of ore, washing, concentration, separation, chemical processes and extraction of pure metal and their alloying to suit various applications, sometimes for direct use as a finished product, but more often in a form that requires further working to achieve the given properties to suit the applications.

Chalcopyrite ( KAL-kə-PY-ryte, -koh-) is a copper iron sulfide mineral and the most abundant copper ore mineral. It has the chemical formula CuFeS2 and crystallizes in the tetragonal system. It has a brassy to golden yellow color and a hardness of 3.5 to 4 on the Mohs scale. Its streak is diagnostic as green-tinged black.

Bornite, also known as peacock ore, is a sulfide mineral with chemical composition Cu5FeS4 that crystallizes in the orthorhombic system (pseudo-cubic).

Chalcanthite (from Ancient Greek χάλκανθον (khálkanthon), from χαλκός (khalkós) 'copper' and ἄνθος (ánthos) 'flower, bloom') is a richly colored blue-green water-soluble sulfate mineral CuSO4·5H2O. It is commonly found in the late-stage oxidation zones of copper deposits. Due to its ready solubility, chalcanthite is more common in arid regions.

Chalcocite, copper(I) sulfide (Cu2S), is an important copper ore mineral. It is opaque and dark gray to black, with a metallic luster. It has a hardness of 2.5–3 on the Mohs scale. It is a sulfide with a monoclinic crystal system.

Covellite is a rare copper sulfide mineral with the formula CuS. This indigo blue mineral is commonly a secondary mineral in limited abundance and although it is not an important ore of copper itself, it is well known to mineral collectors.
Kambalda type komatiitic nickel ore deposits are a class of magmatic iron-nickel-copper-platinum-group element ore deposit in which the physical processes of komatiite volcanology serve to deposit, concentrate and enrich a Fe-Ni-Cu-(PGE) sulfide melt within the lava flow environment of an erupting komatiite volcano.

Digenite is a copper sulfide mineral with formula: Cu9S5. Digenite is a black to dark blue opaque mineral that crystallizes with a trigonal–hexagonal scalenohedral structure. In habit it is usually massive, but does often show pseudo-cubic forms. It has poor to indistinct cleavage and a brittle fracture. It has a Mohs hardness of 2.5 to 3 and a specific gravity of 5.6. It is found in copper sulfide deposits of both primary and supergene occurrences. It is typically associated with and often intergrown with chalcocite, covellite, djurleite, bornite, chalcopyrite and pyrite. The type locality is Sangerhausen, Thuringia, Germany, in copper slate deposits.

Gaspéite, a very rare nickel carbonate mineral, with the formula (Ni,Fe,Mg)CO3, is named for the place it was first described, in the Gaspé Peninsula, Québec, Canada.

Kambaldaite, NaNi4(CO3)3(OH)3·3H2O, is an extremely rare hydrated sodium nickel carbonate mineral described from gossaniferous material associated with Kambalda type komatiitic nickel ore deposits at Kambalda, Western Australia, and Widgie Townsite nickel gossan, Widgiemooltha, Western Australia.
Violarite (Fe2+Ni23+S4) is a supergene sulfide mineral associated with the weathering and oxidation of primary pentlandite nickel sulfide ore minerals.

Polydymite, Ni2+Ni23+S4, is a supergene thiospinel sulfide mineral associated with the weathering of primary pentlandite nickel sulfide.

Concrete degradation may have many different causes. Concrete is mostly damaged by the corrosion of reinforcement bars due to the carbonatation of hardened cement paste or chloride attack under wet conditions. Chemical damage is caused by the formation of expansive products produced by chemical reactions, by aggressive chemical species present in groundwater and seawater, or by microorganisms Other damaging processes can also involve calcium leaching by water infiltration, physical phenomena initiating cracks formation and propagation, fire or radiant heat, aggregate expansion, sea water effects, leaching, and erosion by fast-flowing water.
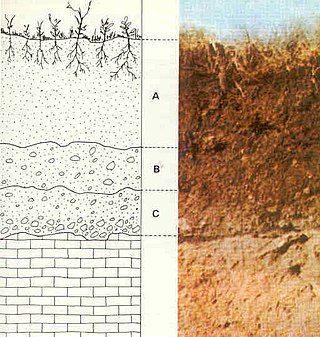
Saprolite is a chemically weathered rock. Saprolites form in the lower zones of soil profiles and represent deep weathering of the bedrock surface. In most outcrops, its color comes from ferric compounds. Deeply weathered profiles are widespread on the continental landmasses between latitudes 35°N and 35°S.
In ore deposit geology, hypogene processes occur deep below the Earth's surface, and tend to form deposits of primary minerals, as opposed to supergene processes that occur at or near the surface, and tend to form secondary minerals.

Rampgill mine is a disused lead mine at Nenthead, Alston Moor, Cumbria, England UK Grid Reference: NY78184351
Polymetallic ores or multimetal ores are complex ores containing a number of chemical elements, among which the most important are lead and zinc. In addition, polymetallic ores can contain copper, gold, silver, cadmium, sometimes bismuth, tin, indium and gallium. The main minerals that form polymetallic ores are galena, sphalerite, to a lesser extent pyrite, chalcopyrite, arsenopyrite, cassiterite. They are most commonly formed from sulfides but also include oxides.
Chvilevaite (Russian: чвилеваи́т, чвилёваи́т, in its own name) is a rare hydrothermal polymetallic mineral from the class of complex sulfides, forming microscopic grains in related minerals, its composition is a rare combination of alkali (combining lithophile) and chalcophile metals — sodium ferro-sulfide, zinc and copper with the calculation formula Na(Cu,Fe,Zn)2S4, originally published and confirmed as Na(Cu,Fe,Zn)2S2.














