
Apocynaceae is a family of flowering plants that includes trees, shrubs, herbs, stem succulents, and vines, commonly known as the dogbane family, because some taxa were used as dog poison. Members of the family are native to the European, Asian, African, Australian, and American tropics or subtropics, with some temperate members. The former family Asclepiadaceae is considered a subfamily of Apocynaceae and contains 348 genera. A list of Apocynaceae genera may be found here.

Tabernanthe iboga (iboga) is an evergreen rainforest shrub native to Central Africa. A member of the Apocynaceae family indigenous to Gabon, the Democratic Republic of Congo, and the Republic of Congo, it is cultivated across Central Africa for its medicinal and other effects.
An oneirogen, from the Greek ὄνειρος óneiros meaning "dream" and gen "to create", is a substance or other stimulus which produces or enhances dreamlike states of consciousness. This is characterized by an immersive dream state similar to REM sleep, which can range from realistic to alien or abstract.

Ibogaine is a psychoactive indole alkaloid obtained either by extraction from plants in the family Apocynaceae such as Tabernanthe iboga, Voacanga africana, and Tabernaemontana undulata or by semi-synthesis from the precursor compound voacangine, another plant alkaloid. The total synthesis of ibogaine was described in 1956. Structural elucidation by X-ray crystallography was completed in 1960.

18-Methoxycoronaridine, also known as zolunicant, is a derivative of ibogaine invented in 1996 by the research team around the pharmacologist Stanley D. Glick from the Albany Medical College and the chemists Upul K. Bandarage and Martin E. Kuehne from the University of Vermont. In animal studies it has proven to be effective at reducing self-administration of morphine, cocaine, methamphetamine, nicotine and sucrose. It has also been shown to produce anorectic effects in obese rats, most likely due to the same actions on the reward system which underlie its anti-addictive effects against drug addiction.

Voacangine is an alkaloid found predominantly in the root bark of the Voacanga africana tree, as well as in other plants such as Tabernanthe iboga, Tabernaemontana africana, Trachelospermum jasminoides, Tabernaemontana divaricata and Ervatamia yunnanensis. It is an iboga alkaloid which commonly serves as a precursor for the semi-synthesis of ibogaine. It has been demonstrated in animals to have similar anti-addictive properties to ibogaine itself. It also potentiates the effects of barbiturates. Under UV-A and UV-B light its crystals fluoresce blue-green, and it is soluble in ethanol.

Indole alkaloids are a class of alkaloids containing a structural moiety of indole; many indole alkaloids also include isoprene groups and are thus called terpene indole or secologanin tryptamine alkaloids. Containing more than 4100 known different compounds, it is one of the largest classes of alkaloids. Many of them possess significant physiological activity and some of them are used in medicine. The amino acid tryptophan is the biochemical precursor of indole alkaloids.

Noribogaine, or 12-hydroxyibogamine, is the principal psychoactive metabolite of the oneirogen ibogaine. It is thought to be involved in the antiaddictive effects of ibogaine-containing plant extracts, such as Tabernanthe iboga.

Coronaridine, also known as 18-carbomethoxyibogamine, is an alkaloid found in Tabernanthe iboga and related species, including Tabernaemontana divaricata for which it was named.

Ibogamine is an anti-convulsant, anti-addictive, CNS stimulant alkaloid found in Tabernanthe iboga and Crepe Jasmine. Basic research related to how addiction affects the brain has used this chemical.

Tabernanthine is an alkaloid found in Tabernanthe iboga.
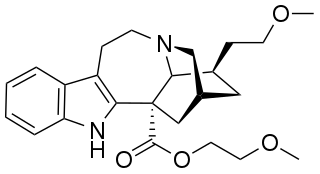
(−)-2-Methoxyethyl-18-methoxycoronaridinate (ME-18-MC) is a second generation synthetic derivative of ibogaine developed by the research team led by the pharmacologist Stanley D. Glick from the Albany Medical College and the chemist Martin E. Kuehne from the University of Vermont. In animal studies it has shown similar efficacy to the related compound 18-methoxycoronaridine (18-MC) at reducing self-administration of morphine and methamphetamine but with higher potency by weight, showing anti-addictive effects at the equivalent of half the minimum effective dose of 18-MC. Similarly to 18-MC itself, ME-18-MC acts primarily as a selective α3β4 nicotinic acetylcholine antagonist, although it has a slightly stronger effect than 18-MC as an NMDA antagonist, and its effects on opioid receptors are weaker than those of 18-MC at all except the kappa opioid receptor, at which it has slightly higher affinity than 18-MC.

Voacamine, also known under the older names voacanginine and vocamine, is a naturally occurring dimeric indole alkaloid of the secologanin type, found in a number of plants, including Voacanga africana and Tabernaemontana divaricata. It is approved for use as an antimalarial drug in several African countries. Voacamine exhibits cannabinoid CB1 receptor antagonistic activity.
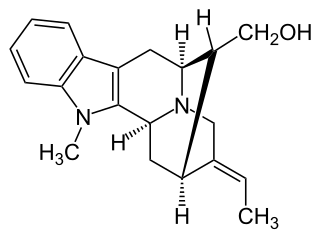
Affinisine is a monoterpenoid indole alkaloid which can be isolated from plants of the genus Tabernaemontana. Structurally, it can be considered a member of the sarpagine alkaloid family and may be synthesized from tryptophan via a Pictet-Spengler reaction.
Iboga-type alkaloids are a set of monoterpene indole alkaloids comprising naturally occurring compounds found in Tabernanthe and Tabernaemontana, as well as synthetic structural analogs. Naturally occurring iboga-type alkaloids include ibogamine, ibogaine, tabernanthine, and other substituted ibogamines. Many iboga-type alkaloids display biological activities such as cardiac toxicity and psychoactive effects, and some have been studied as potential treatments for drug addiction.

Tabernaemontanine is a naturally occurring monoterpene indole alkaloid found in several species in the genus Tabernaemontana including Tabernaemontana divaricata.

Voacristine is a indole alkaloid occurring in Voacanga and Tabernaemontana genus. It is also an iboga type alkaloid.

19,20-Dihydroervahanine A is an alkaloid, a natural product which is found in the root of the Southeast Asian plant Tabernaemontana divaricata. It inhibits acetylcholinesterase in vitro more potently than galantamine.

Conopharyngine is the major alkaloid present in the leaves and stem-bark of Tabernaemontana pachysiphon and Conopharyngia durissima. It is closely related voacangine and coronaridine. Conopharyngine pseudoindoxyl, a derivative of it, is also found in the same plant Tabernaemontana pachysiphon.

















