In organic chemistry, the cycloalkanes are the monocyclic saturated hydrocarbons. In other words, a cycloalkane consists only of hydrogen and carbon atoms arranged in a structure containing a single ring, and all of the carbon-carbon bonds are single. The larger cycloalkanes, with more than 20 carbon atoms are typically called cycloparaffins. All cycloalkanes are isomers of alkenes.

In organic chemistry, aromaticity is a chemical property describing the way in which a conjugated ring of unsaturated bonds, lone pairs, or empty orbitals exhibits a stabilization stronger than would be expected by the stabilization of conjugation alone. The earliest use of the term was in an article by August Wilhelm Hofmann in 1855. There is no general relationship between aromaticity as a chemical property and the olfactory properties of such compounds.
Cubane is a synthetic hydrocarbon compound with the formula C8H8. It consists of eight carbon atoms arranged at the corners of a cube, with one hydrogen atom attached to each carbon atom. A solid crystalline substance, cubane is one of the Platonic hydrocarbons and a member of the prismanes. It was first synthesized in 1964 by Philip Eaton and Thomas Cole. Before this work, Eaton believed that cubane would be impossible to synthesize due to the "required 90 degree bond angles". The cubic shape requires the carbon atoms to adopt an unusually sharp 90° bonding angle, which would be highly strained as compared to the 109.45° angle of a tetrahedral carbon. Once formed, cubane is quite kinetically stable, due to a lack of readily available decomposition paths. It is the simplest hydrocarbon with octahedral symmetry.
The Friedel–Crafts reactions are a set of reactions developed by Charles Friedel and James Crafts in 1877 to attach substituents to an aromatic ring. Friedel–Crafts reactions are of two main types: alkylation reactions and acylation reactions. Both proceed by electrophilic aromatic substitution.

Louis Frederick Fieser was an American organic chemist, professor, and in 1968, professor emeritus at Harvard University. His award-winning research included work on blood-clotting agents including the first synthesis of vitamin K, synthesis and screening of quinones as antimalarial drugs, work with steroids leading to the synthesis of cortisone, and study of the nature of polycyclic aromatic hydrocarbons. He also invented militarily effective napalm while at Harvard in 1942.
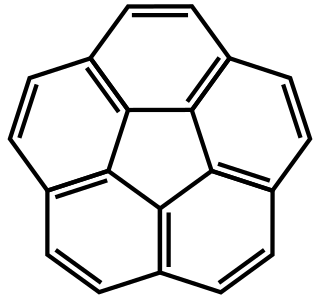
Corannulene is a polycyclic aromatic hydrocarbon with chemical formula C20H10. The molecule consists of a cyclopentane ring fused with 5 benzene rings, so another name for it is [5]circulene. It is of scientific interest because it is a geodesic polyarene and can be considered a fragment of buckminsterfullerene. Due to this connection and also its bowl shape, corannulene is also known as a buckybowl. Buckybowls are fragments of buckyballs. Corannulene exhibits a bowl-to-bowl inversion with an inversion barrier of 10.2 kcal/mol (42.7 kJ/mol) at −64 °C.

Prismane or 'Ladenburg benzene' is a polycyclic hydrocarbon with the formula C6H6. It is an isomer of benzene, specifically a valence isomer. Prismane is far less stable than benzene. The carbon (and hydrogen) atoms of the prismane molecule are arranged in the shape of a six-atom triangular prism—this compound is the parent and simplest member of the prismanes class of molecules. Albert Ladenburg proposed this structure for the compound now known as benzene. The compound was not synthesized until 1973.

Pentacene is a polycyclic aromatic hydrocarbon consisting of five linearly-fused benzene rings. This highly conjugated compound is an organic semiconductor. The compound generates excitons upon absorption of ultra-violet (UV) or visible light; this makes it very sensitive to oxidation. For this reason, this compound, which is a purple powder, slowly degrades upon exposure to air and light.

Fluorene, or 9H-fluorene is an organic compound with the formula (C6H4)2CH2. It forms white crystals that exhibit a characteristic, aromatic odor similar to that of naphthalene. Despite its name, it does not contain the element fluorine, but rather it comes from the violet fluorescence it exhibits. For commercial purposes it is obtained from coal tar, where it was discovered and named by Marcellin Berthelot in 1867.

Hexacene is an aromatic compound consisting of six linearly-fused benzene rings. It is a blue-green, air-stable solid with low solubility.

A circulene is a macrocyclic arene in which a central polygon is surrounded and fused by benzenoids. Nomenclature within this class of molecules is based on the number of benzene rings surrounding the core, which is equivalent to the size of the central polygon. Examples which have been synthesized include [5]circulene (corannulene), [6]circulene (coronene), [7]circulene, and [12]circulene (kekulene) These compounds belong to a larger class of geodesic polyarenes. Whereas [5]circulene is bowl-shaped and [6]circulene is planar, [7]circulene has a unique saddle-shaped structure. The helicenes are a conceptually related class of structures in which the array of benzene rings form an open helix rather than a closed ring.
[n]Radialenes are alicyclic organic compounds containing n cross-conjugated exocyclic double bonds. The double bonds are commonly alkene groups but those with a carbonyl (C=O) group are also called radialenes. For some members the unsubstituted parent radialenes are elusive but many substituted derivatives are known.

A fenestrane in organic chemistry is a type of chemical compound with a central quaternary carbon atom which serves as a common vertex for four fused carbocycles. They can be regarded as spiro compounds twice over. Because of their inherent strain and instability, fenestranes are of theoretical interest to chemists. The name—proposed in 1972 by Vlasios Georgian and Martin Saltzman—is derived from the Latin word for window, fenestra. Georgian had intended that "fenestrane" solely referred to [4.4.4.4]fenestrane, whose skeletal structure looks like windows, and Kenneth B. Wiberg called that specific structure "windowpane". The term fenestrane has since become generalized to refer to the whole class of molecules that have various other ring-sizes. Georgian recommended rosettane for the class, based on the structural appearance as a rosette of flowers.

Biphenylene is an organic compound with the formula (C6H4)2. It is a pale, yellowish solid with a hay-like odor. Despite its unusual structure, it behaves like a traditional polycyclic aromatic hydrocarbon.

Tetraphenylcyclopentadienone is an organic compound with the formula (C6H5C)4C4C=O. It is classified as a cyclic dienone. It is a dark purple to black crystalline solid that is soluble in organic solvents. It is an easily made building block for many organic and organometallic compounds.

Basketane is a polycyclic alkane with the chemical formula C10H12. The name is taken from its structural similarity to a basket shape. Basketane was first synthesized in 1966, independently by Masamune and Dauben and Whalen. A patent application published in 1988 used basketane, which is a hydrocarbon, as a source material in doping thin diamond layers because of the molecule's high vapor pressure, carbon ring structure, and fewer hydrogen-to-carbon bond ratio.

In organic chemistry, the Mallory reaction is a photochemical-cyclization–elimination reaction of diaryl-ethylene structures to form phenanthrenes and other polycyclic form polycyclic aromatic hydrocarbons and heteroaromatics. This name reaction is named for Frank Mallory, who discovered it while a graduate student.

Olympicene is an organic carbon-based molecule formed of five rings, of which four are benzene rings, joined in the shape of the Olympic rings.
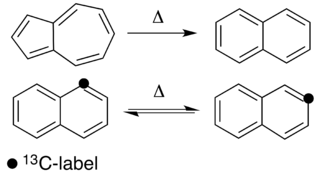
Thermal rearrangements of aromatic hydrocarbons are considered to be unimolecular reactions that directly involve the atoms of an aromatic ring structure and require no other reagent than heat. These reactions can be categorized in two major types: one that involves a complete and permanent skeletal reorganization (isomerization), and one in which the atoms are scrambled but no net change in the aromatic ring occurs (automerization). The general reaction schemes of the two types are illustrated in Figure 1.
The [4+4] Photocycloaddition is a cycloaddition reaction in which two unsaturated molecules connect via four atoms from each molecule to create an eight-membered ring. As a photochemical reaction, it is promoted by some form of light, as opposed to a thermal process.