Bioaccumulation is the gradual accumulation of substances, such as pesticides or other chemicals, in an organism. Bioaccumulation occurs when an organism absorbs a substance faster than it can be lost or eliminated by catabolism and excretion. Thus, the longer the biological half-life of a toxic substance, the greater the risk of chronic poisoning, even if environmental levels of the toxin are not very high. Bioaccumulation, for example in fish, can be predicted by models. Hypothesis for molecular size cutoff criteria for use as bioaccumulation potential indicators are not supported by data. Biotransformation can strongly modify bioaccumulation of chemicals in an organism.

Aconitine is an alkaloid toxin produced by various plant species belonging to the genus Aconitum, known also commonly by the names wolfsbane and monkshood. Monkshood is notorious for its toxic properties.

Ichthyothere is a genus of flowering plants, found in parts of South America and Central America.

Batrachotoxin (BTX) is an extremely potent cardio- and neurotoxic steroidal alkaloid found in certain species of beetles, birds, and frogs. The name is from the Greek word βάτραχος, bátrachos, 'frog'. Structurally-related chemical compounds are often referred to collectively as batrachotoxins. In certain frogs, this alkaloid is present mostly on the skin. Such frogs are among those used for poisoning darts. Batrachotoxin binds to and irreversibly opens the sodium channels of nerve cells and prevents them from closing, resulting in paralysis and death. No antidote is known.
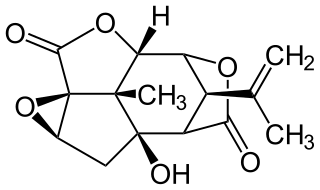
Picrotoxin, also known as cocculin, is a poisonous crystalline plant compound. It was first isolated by the French pharmacist and chemist Pierre François Guillaume Boullay (1777–1869) in 1812. The name "picrotoxin" is a combination of the Greek words "picros" (bitter) and "toxicon" (poison). A mixture of two different compounds, picrotoxin occurs naturally in the fruit of the Anamirta cocculus plant, although it can also be synthesized chemically.

Saxitoxin (STX) is a potent neurotoxin and the best-known paralytic shellfish toxin (PST). Ingestion of saxitoxin by humans, usually by consumption of shellfish contaminated by toxic algal blooms, is responsible for the illness known as paralytic shellfish poisoning (PSP).

The Pauson–Khand reaction is a chemical reaction described as a [2+2+1] cycloaddition between an alkyne, an alkene and carbon monoxide to form a α,β-cyclopentenone. Ihsan Ullah Khand (1935-1980) discovered the reaction around 1970, while working as a postdoctoral associate with Peter Ludwig Pauson (1925–2013) at the University of Strathclyde in Glasgow. Pauson and Khand's initial findings were intermolecular in nature, but starting a decade after the reaction's discovery, many intramolecular examples have been highlighted in both synthesis and methodology reports. This reaction was originally mediated by stoichiometric amounts of dicobalt octacarbonyl, but newer versions are both more efficient, enhancing reactivity and yield via utilizing different chiral auxiliaries for stereo induction, main group transition-metals, and additives.
Okadaic acid, C44H68O13, is a toxin produced by several species of dinoflagellates, and is known to accumulate in both marine sponges and shellfish. One of the primary causes of diarrhetic shellfish poisoning, okadaic acid is a potent inhibitor of specific protein phosphatases and is known to have a variety of negative effects on cells. A polyketide, polyether derivative of a C38 fatty acid, okadaic acid and other members of its family have shined light upon many biological processes both with respect to dinoflagellete polyketide synthesis as well as the role of protein phosphatases in cell growth.

Cicutoxin is a naturally-occurring poisonous chemical compound produced by several plants from the family Apiaceae including water hemlock (Cicuta species) and water dropwort (Oenanthe crocata). The compound contains polyene, polyyne, and alcohol functional groups and is a structural isomer of oenanthotoxin, also found in water dropwort. Both of these belong to the C17-polyacetylenes chemical class.

A polyyne is any organic compound with alternating single and triple bonds; that is, a series of consecutive alkynes, (−C≡C−)n with n greater than 1. These compounds are also called polyacetylenes, especially in the natural products and chemical ecology literature, even though this nomenclature more properly refers to acetylene polymers composed of alternating single and double bonds (−CR=CR′−)n with n greater than 1. They are also sometimes referred to as oligoynes, or carbinoids after "carbyne" (−C≡C−)∞, the hypothetical allotrope of carbon that would be the ultimate member of the series. The synthesis of this substance has been claimed several times since the 1960s, but those reports have been disputed. Indeed, the substances identified as short chains of "carbyne" in many early organic synthesis attempts would be called polyynes today.

Silver acetate is a coordination compound with the empirical formula CH3CO2Ag (or AgC2H3O2). A photosensitive, white, crystalline solid, it is a useful reagent in the laboratory as a source of silver ions lacking an oxidizing anion.
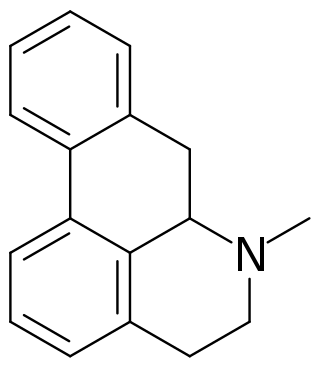
Aporphine is an alkaloid with the chemical formula C17H17N. It is the core chemical substructure of the aporphine alkaloids, a subclass of quinoline alkaloids. It can exist in either of two enantiomeric forms, (R)-aporphine and (S)-aporphine.

Brevetoxin (PbTx), or brevetoxins, are a suite of cyclic polyether compounds produced naturally by a species of dinoflagellate known as Karenia brevis. Brevetoxins are neurotoxins that bind to voltage-gated sodium channels in nerve cells, leading to disruption of normal neurological processes and causing the illness clinically described as neurotoxic shellfish poisoning (NSP).

Dichapetalum cymosum, commonly known as gifblaar from Afrikaans, or occasionally by its English translation, poison leaf, is a small prostrate shrub occurring in northern parts of Southern Africa in the family Dichapetalaceae. It is notable as a common cause of lethal cattle poisoning in this region and is considered one of the 'big 6' toxic plants of cattle in South Africa. A 1996 estimate of plant poisonings in South Africa attributes 8% of cattle mortality caused by poisonous plants to it. The majority (70%) of fatal cases are in Limpopo province, with 10% each in North West, Mpumalanga, and Gauteng. Fluoroacetate, the poison used to synthetically produce Compound 1080 used extensively in New Zealand, occurs in all parts of the plant and is responsible for the toxic effects shown.

Ptaquiloside is a norsesquiterpene glucoside produced by bracken ferns during metabolism. It is identified to be the main carcinogen of the ferns and to be responsible for their biological effects, such as haemorrhagic disease and bright blindness in livestock and oesophageal, gastric cancer in humans. Ptaquiloside has unstable chemical structure and acts as a DNA alkylating agent under physiological conditions. It was first isolated and characterized by Yamada and co-workers in 1983.
The Saegusa–Ito oxidation is a chemical reaction used in organic chemistry. It was discovered in 1978 by Takeo Saegusa and Yoshihiko Ito as a method to introduce α-β unsaturation in carbonyl compounds. The reaction as originally reported involved formation of a silyl enol ether followed by treatment with palladium(II) acetate and benzoquinone to yield the corresponding enone. The original publication noted its utility for regeneration of unsaturation following 1,4-addition with nucleophiles such as organocuprates.

(−)-Magellanine is a member of the Lycopodium alkaloid class of natural products. It was isolated from the club moss Lycopodium magellanicum in 1976. It has been synthesized five times, with the first synthesis having been completed by the Larry E. Overman group at the University of California, Irvine in 1993. It has also been synthesized by the Leo Paquette group in 1993 at Ohio State University, the Chun-Chen Liao group in 2002 at National Tsing Hua University, the Miyuki Ishikazi and Tamiko Takahashi groups in 2005 at the Josai International University and Tokyo University of Science, and the Chisato Mukai group in 2007 at the Kanazawa University. One partial synthesis was completed by the A. I. Meyers group in 1995 at Colorado State University.

Gelsemine (C20H22N2O2) is an indole alkaloid isolated from flowering plants of the genus Gelsemium, a plant native to the subtropical and tropical Americas, and southeast Asia, and is a highly toxic compound that acts as a paralytic, exposure to which can result in death. It has generally potent activity as an agonist of the mammalian glycine receptor, the activation of which leads to an inhibitory postsynaptic potential in neurons following chloride ion influx, and systemically, to muscle relaxation of varying intensity and deleterious effect. Despite its danger and toxicity, recent pharmacological research has suggested that the biological activities of this compound may offer opportunities for developing treatments related to xenobiotic or diet-induced oxidative stress, and of anxiety and other conditions, with ongoing research including attempts to identify safer derivatives and analogs to make use of gelsemine's beneficial effects.

Juncusol is a 9,10-dihydrophrenathrene found in Juncus species such as J. acutus, J. effusus or J. roemerianus.
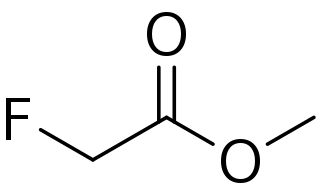
Methyl fluoroacetate (MFA) is an extremely toxic methyl ester of fluoroacetic acid. It is a colorless, odorless liquid at room temperature. It is used as a laboratory chemical and as a rodenticide. Because of its extreme toxicity, MFA was studied for potential use as a chemical weapon. The general population is not likely to be exposed to methyl fluoroacetate. People who use MFA for work, however, can breathe in or have direct skin contact with the substance.

















