
Proteinase 3, also known as PRTN3, is an enzyme that in humans is encoded by the PRTN3 gene.
Mothers against decapentaplegic homolog 2 also known as SMAD family member 2 or SMAD2 is a protein that in humans is encoded by the SMAD2 gene. MAD homolog 2 belongs to the SMAD, a family of proteins similar to the gene products of the Drosophila gene 'mothers against decapentaplegic' (Mad) and the C. elegans gene Sma. SMAD proteins are signal transducers and transcriptional modulators that mediate multiple signaling pathways.

Cathepsin G is a protein that in humans is encoded by the CTSG gene. It is one of the three serine proteases of the chymotrypsin family that are stored in the azurophil granules, and also a member of the peptidase S1 protein family. Cathepsin G plays an important role in eliminating intracellular pathogens and breaking down tissues at inflammatory sites, as well as in anti-inflammatory response.
C-X-C motif chemokine 5 is a protein that in humans is encoded by the CXCL5 gene.

Cathelicidin antimicrobial peptide (CAMP) is a polypeptide that is primarily stored in the lysosomes of macrophages and polymorphonuclear leukocytes (PMNs); in humans, the CAMP gene encodes the peptide precursor CAP-18, which is processed by proteinase 3-mediated extracellular cleavage into the active form LL-37. LL-37 is the only peptide in the Cathelicidin family found in the human body.

Chemokine ligand 7 (CXCL7) is a human gene.

Interleukin 8 receptor, beta is a chemokine receptor. IL8RB is also known as CXCR2, and CXCR2 is now the IUPHAR Committee on Receptor Nomenclature and Drug classification-recommended name.

Dual specificity mitogen-activated protein kinase kinase 2 is an enzyme that in humans is encoded by the MAP2K2 gene. It is more commonly known as MEK2, but has many alternative names including CFC4, MKK2, MAPKK2 and PRKMK2.

Interleukin 8 receptor, alpha is a chemokine receptor. This name and the corresponding gene symbol IL8RA have been replaced by the HGNC approved name C-X-C motif chemokine receptor 1 and the approved symbol CXCR1. It has also been designated as CD181. The IUPHAR Committee on Receptor Nomenclature and Drug Classification use the HGNC recommended name, CXCR1.

Caspase-10 is an enzyme that, in humans, is encoded by the CASP10 gene.

N-formyl peptide receptor 2 (FPR2) is a G-protein coupled receptor (GPCR) located on the surface of many cell types of various animal species. The human receptor protein is encoded by the FPR2 gene and is activated to regulate cell function by binding any one of a wide variety of ligands including not only certain N-Formylmethionine-containing oligopeptides such as N-Formylmethionine-leucyl-phenylalanine (FMLP) but also the polyunsaturated fatty acid metabolite of arachidonic acid, lipoxin A4 (LXA4). Because of its interaction with lipoxin A4, FPR2 is also commonly named the ALX/FPR2 or just ALX receptor.

Casein kinase II subunit beta is a protein that in humans is encoded by the CSNK2B gene.

Low affinity immunoglobulin gamma Fc region receptor II-a is a protein that in humans is encoded by the FCGR2A gene.

Defensin, alpha 1 also known as human alpha defensin 1, human neutrophil peptide 1 (HNP-1) or neutrophil defensin 1 is a human protein that is encoded by the DEFA1 gene. Human alpha defensin 1 belongs to the alpha defensin family of antimicrobial peptides.
Azurocidin also known as cationic antimicrobial protein CAP37 or heparin-binding protein (HBP) is a protein that in humans is encoded by the AZU1 gene.

Arthropod defensins are a family defensin proteins found in mollusks, insects, and arachnids. These cysteine-rich antibacterial peptides are primarily active against Gram-positive bacteria and fungi in vitro. However Drosophila fruit flies mutant for the fly defensin were more susceptible to infection by the Gram-negative bacteria Providencia burhodogranariea, and resisted infection against Gram-positive bacteria like wild-type flies. It remains to be seen how in vitro activity relates to in vivo function. Mutants for the defensin-like antimicrobial peptide Drosomycin were more susceptible to fungi, validating a role for defensin-like peptides in anti-fungal defence.
Defensin, alpha 4 (DEFA4), also known as neutrophil defensin 4 or HNP4, is a human defensin peptide that is encoded by the DEFA4 gene. HNP4 is expressed in the granules of the neutrophil where it defends the host against bacteria and viruses.

Formyl peptide receptor 1 is a cell surface receptor protein that in humans is encoded by the formyl peptide receptor 1 (FPR1) gene. This gene encodes a G protein-coupled receptor cell surface protein that binds and is activated by N-Formylmethionine-containing oligopeptides, particularly N-Formylmethionine-leucyl-phenylalanine (FMLP). FPR1 is prominently expressed by mammalian phagocytic and blood leukocyte cells where it functions to mediate these cells' responses to the N-formylmethionine-containing oligopeptides which are released by invading microorganisms and injured tissues. FPR1 directs these cells to sites of invading pathogens or disrupted tissues and then stimulates these cells to kill the pathogens or to remove tissue debris; as such, it is an important component of the innate immune system that operates in host defense and damage control.

Peptidoglycan recognition proteins (PGRPs) are a group of highly conserved pattern recognition receptors with at least one peptidoglycan recognition domain capable of recognizing the peptidoglycan component of the cell wall of bacteria. They are present in insects, mollusks, echinoderms and chordates. The mechanism of action of PGRPs varies between taxa. In insects, PGRPs kill bacteria indirectly by activating one of four unique effector pathways: prophenoloxidase cascade, Toll pathway, IMD pathway, and induction of phagocytosis. In mammals, PGRPs either kill bacteria directly by interacting with their cell wall or outer membrane, or hydrolyze peptidoglycan. They also modulate inflammation and microbiome and interact with host receptors.
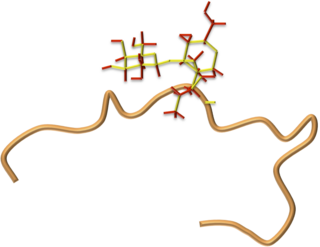
Drosocin is a 19-residue long antimicrobial peptide (AMP) of flies first isolated in the fruit fly Drosophila melanogaster, and later shown to be conserved throughout the genus Drosophila. Drosocin is regulated by the NF-κB Imd signalling pathway in the fly.


















