 | |
| Names | |
|---|---|
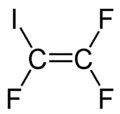
| Preferred IUPAC name 1,1,2-Trifluoro-2-iodoethene | |
| Other names 1,1,2-Trifluoro-2-iodoethylene, trifluoroiodoethylene, iodotrifluoroethylene | |
| Identifiers | |
3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.006.028 |
| EC Number |
|
PubChem CID | |
| UNII | |
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
| C2F3I | |
| Molar mass | 207.92 g/mol |
| Density | 2.284 g/cm3 |
| Boiling point | 30 °C (86 °F; 303 K) |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards | Irritant (Xi) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Iodotrifluoroethylene is the organofluorine compound with the formula C
2F
3I. It is a volatile colorless liquid.