Related Research Articles

Necrosis is a form of cell injury which results in the premature death of cells in living tissue by autolysis. The term "necrosis" came about in the mid-19th century and is commonly attributed to German pathologist Rudolf Virchow in, who is often regarded as one of the founders of modern pathology. Necrosis is caused by factors external to the cell or tissue, such as infection, or trauma which result in the unregulated digestion of cell components. In contrast, apoptosis is a naturally occurring programmed and targeted cause of cellular death. While apoptosis often provides beneficial effects to the organism, necrosis is almost always detrimental and can be fatal.
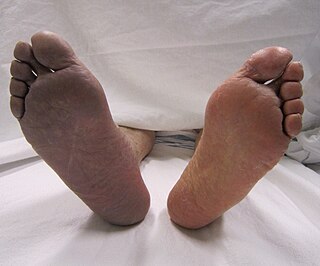
Thrombosis is the formation of a blood clot inside a blood vessel, obstructing the flow of blood through the circulatory system. When a blood vessel is injured, the body uses platelets (thrombocytes) and fibrin to form a blood clot to prevent blood loss. Even when a blood vessel is not injured, blood clots may form in the body under certain conditions. A clot, or a piece of the clot, that breaks free and begins to travel around the body is known as an embolus.

Ischemia or ischaemia is a restriction in blood supply to any tissue, muscle group, or organ of the body, causing a shortage of oxygen that is needed for cellular metabolism. Ischemia is generally caused by problems with blood vessels, with resultant damage to or dysfunction of tissue i.e. hypoxia and microvascular dysfunction. It also implies local hypoxia in a part of a body resulting from constriction. Ischemia causes not only insufficiency of oxygen, but also reduced availability of nutrients and inadequate removal of metabolic wastes. Ischemia can be partial or total blockage. The inadequate delivery of oxygenated blood to the organs must be resolved either by treating the cause of the inadequate delivery or reducing the oxygen demand of the system that needs it. For example, patients with myocardial ischemia have a decreased blood flow to the heart and are prescribed with medications that reduce chronotrophy and ionotrophy to meet the new level of blood delivery supplied by the stenosed vasculature so that it is adequate.
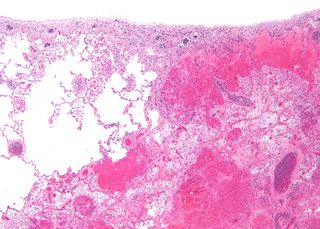
Infarction is tissue death (necrosis) due to inadequate blood supply to the affected area. It may be caused by artery blockages, rupture, mechanical compression, or vasoconstriction. The resulting lesion is referred to as an infarct (from the Latin infarctus, "stuffed into").

Reperfusion injury, sometimes called ischemia-reperfusion injury (IRI) or reoxygenation injury, is the tissue damage caused when blood supply returns to tissue after a period of ischemia or lack of oxygen. The absence of oxygen and nutrients from blood during the ischemic period creates a condition in which the restoration of circulation results in inflammation and oxidative damage through the induction of oxidative stress rather than restoration of normal function.

Lipid emulsion or fat emulsion refers to an emulsion of fat for human intravenous use, to administer nutrients to critically-ill patients that cannot consume food. It is often referred to by the brand name of the most commonly used version, Intralipid, which is an emulsion containing soybean oil, egg phospholipids and glycerin, and is available in 10%, 20% and 30% concentrations. The 30% concentration is not approved for direct intravenous infusion, but should be mixed with amino acids and dextrose as part of a total nutrient admixture.

Crush syndrome is a medical condition characterized by major shock and kidney failure after a crushing injury to skeletal muscle. Crush injury is compression of the arms, legs, or other parts of the body that causes muscle swelling and/or neurological disturbances in the affected areas of the body, while crush syndrome is localized crush injury with systemic manifestations. Cases occur commonly in catastrophes such as earthquakes, to individuals that have been trapped under fallen or moving masonry.
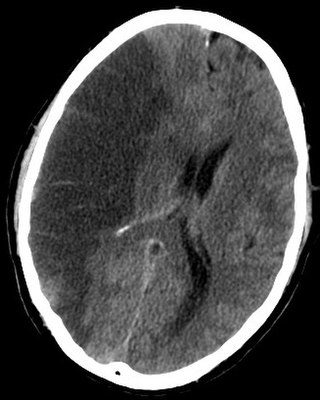
Brain ischemia is a condition in which there is insufficient bloodflow to the brain to meet metabolic demand. This leads to poor oxygen supply or cerebral hypoxia and thus leads to the death of brain tissue or cerebral infarction/ischemic stroke. It is a sub-type of stroke along with subarachnoid hemorrhage and intracerebral hemorrhage.
Animal models of ischemic stroke are procedures inducing cerebral ischemia. The aim is the study of basic processes or potential therapeutic interventions in this disease, and the extension of the pathophysiological knowledge on and/or the improvement of medical treatment of human ischemic stroke. Ischemic stroke has a complex pathophysiology involving the interplay of many different cells and tissues such as neurons, glia, endothelium, and the immune system. These events cannot be mimicked satisfactorily in vitro yet. Thus a large portion of stroke research is conducted on animals.

Ischemic colitis is a medical condition in which inflammation and injury of the large intestine result from inadequate blood supply. Although uncommon in the general population, ischemic colitis occurs with greater frequency in the elderly, and is the most common form of bowel ischemia. Causes of the reduced blood flow can include changes in the systemic circulation or local factors such as constriction of blood vessels or a blood clot. In most cases, no specific cause can be identified.
Ischemic preconditioning (IPC) is an experimental technique for producing resistance to the loss of blood supply, and thus oxygen, to tissues of many types. In the heart, IPC is an intrinsic process whereby repeated short episodes of ischaemia protect the myocardium against a subsequent ischaemic insult. It was first identified in 1986 by Murry et al. This group exposed anesthetised open-chest dogs to four periods of 5 minute coronary artery occlusions followed by a 5-minute period of reperfusion before the onset of a 40-minute sustained occlusion of the coronary artery. The control animals had no such period of “ischaemic preconditioning” and had much larger infarct sizes compared with the dogs that did. The exact molecular pathways behind this phenomenon have yet to be fully understood.

Translocator protein (TSPO) is an 18 kDa protein mainly found on the outer mitochondrial membrane. It was first described as peripheral benzodiazepine receptor (PBR), a secondary binding site for diazepam, but subsequent research has found the receptor to be expressed throughout the body and brain. In humans, the translocator protein is encoded by the TSPO gene. It belongs to a family of tryptophan-rich sensory proteins. Regarding intramitochondrial cholesterol transport, TSPO has been proposed to interact with StAR to transport cholesterol into mitochondria, though evidence is mixed.
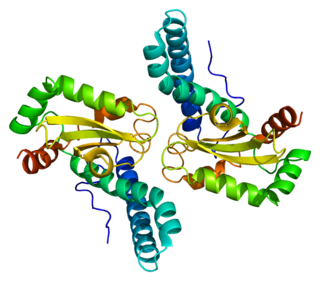
Superoxide dismutase 2, mitochondrial (SOD2), also known as manganese-dependent superoxide dismutase (MnSOD), is an enzyme which in humans is encoded by the SOD2 gene on chromosome 6. A related pseudogene has been identified on chromosome 1. Alternative splicing of this gene results in multiple transcript variants. This gene is a member of the iron/manganese superoxide dismutase family. It encodes a mitochondrial protein that forms a homotetramer and binds one manganese ion per subunit. This protein binds to the superoxide byproducts of oxidative phosphorylation and converts them to hydrogen peroxide and diatomic oxygen. Mutations in this gene have been associated with idiopathic cardiomyopathy (IDC), premature aging, sporadic motor neuron disease, and cancer.

Acadesine (INN), also known as 5-aminoimidazole-4-carboxamide-1-β-D-ribofuranoside, AICA-riboside, and AICAR, is an AMP-activated protein kinase activator which is used for the treatment of acute lymphoblastic leukemia and may have applications in treating other disorders such as diabetes. AICAR has been used clinically to treat and protect against cardiac ischemic injury. The drug was first used in the 1980s as a method to preserve blood flow to the heart during surgery.

Voltage-dependent anion-selective channel protein 2 is a protein that in humans is encoded by the VDAC2 gene on chromosome 10. This protein is a voltage-dependent anion channel and shares high structural homology with the other VDAC isoforms. VDACs are generally involved in the regulation of cell metabolism, mitochondrial apoptosis, and spermatogenesis. Additionally, VDAC2 participates in cardiac contractions and pulmonary circulation, which implicate it in cardiopulmonary diseases. VDAC2 also mediates immune response to infectious bursal disease (IBD).

Voltage-dependent anion-selective channel protein 3 (VDAC3) is a protein that in humans is encoded by the VDAC3 gene on chromosome 8. The protein encoded by this gene is a voltage-dependent anion channel and shares high structural homology with the other VDAC isoforms. Nonetheless, VDAC3 demonstrates limited pore-forming ability and, instead, interacts with other proteins to perform its biological functions, including sperm flagella assembly and centriole assembly. Mutations in VDAC3 have been linked to male infertility, as well as Parkinson’s disease.

Rottlerin (mallotoxin) is a polyphenol natural product isolated from the Asian tree Mallotus philippensis. Rottlerin displays a complex spectrum of pharmacology.
Cardioprotection includes all mechanisms and means that contribute to the preservation of the heart by reducing or even preventing myocardial damage. Cardioprotection encompasses several regimens that have shown to preserve function and viability of cardiac muscle cell tissue subjected to ischemic insult or reoxygenation. Cardioprotection includes strategies that are implemented before an ischemic event, during an ischemic event and after the event and during reperfusion. These strategies can be further stratified by performing the intervention locally or remotely, creating classes of conditioning known as remote ischemic PC (RIPC), remote ischemic PostC and remote ischemic PerC. Classical (local) preconditioning has an early phase with an immediate onset lasting 2–3 hours that protects against myocardial infarction. The early phase involves post-translational modification of preexisting proteins, brought about by the activation of G protein-coupled receptors as well as downstream MAPK's and PI3/Akt. These signaling events act on the ROS-generating mitochondria, activate PKCε and the Reperfusion Injury Salvage Kinase (RISK) pathway, preventing mitochondrial permeability transition pore (MTP) opening. The late phase with an onset of 12–24 hours that lasts 3–4 days and protects against both infarction and reversible postischemic contractile dysfunction, termed myocardial stunning. This phase involves the synthesis of new cardioprotective proteins stimulated by nitric oxide (NO), ROS and adenosine acting on kinases such as PKCε and Src, which in turn activate gene transcription and upregulation of late PC molecular players.
Kidney ischemia is a disease with a high morbidity and mortality rate. Blood vessels shrink and undergo apoptosis which results in poor blood flow in the kidneys. More complications happen when failure of the kidney functions result in toxicity in various parts of the body which may cause septic shock, hypovolemia, and a need for surgery. What causes kidney ischemia is not entirely known, but several pathophysiology relating to this disease have been elucidated. Possible causes of kidney ischemia include the activation of IL-17C and hypoxia due to surgery or transplant. Several signs and symptoms include injury to the microvascular endothelium, apoptosis of kidney cells due to overstress in the endoplasmic reticulum, dysfunctions of the mitochondria, autophagy, inflammation of the kidneys, and maladaptive repair.
Roberta Anne Gottlieb is an American oncologist, academic, and researcher. She is a Professor, and Vice-Chair of Translational Medicine in the Department of Biomedical Sciences at Cedars-Sinai Medical Center, and a Professor of Medicine at the University of California, Los Angeles.
References
- ↑ Eltzschig, H.K. & T. Eckle (2011). "Ischemia and reperfusion—from mechanism to translation". Nat Med. 17 (11): 1391–401. doi:10.1038/nm.2507. PMC 3886192 . PMID 22064429.
- ↑ Zuk, A. & J.V. Bonventre (2016). "Acute Kidney Injury". Annu Rev Med. 67: 293–307. doi:10.1146/annurev-med-050214-013407. PMC 4845743 . PMID 26768243.
- ↑ Zager, R.A. & A.C. Johnson (2009). "Renal ischemia-reperfusion injury upregulates histone-modifying enzyme systems and alters histone expression at proinflammatory/profibrotic genes". Am J Physiol Renal Physiol. 296 (5): F1032–F1041. doi:10.1152/ajprenal.00061.2009. PMC 2681356 . PMID 19261745.
- ↑ Pincez, T.; et al. (2016). "[Pulmonary complications of sickle cell disease in children]". Arch Pediatr. 23 (10): 1094–1106. doi:10.1016/j.arcped.2016.06.014. PMID 27642150.
- ↑ Barritault, D.; et al. (2016). "RGTA®-based matrix therapy – A new branch of regenerative medicine in locomotion". Joint Bone Spine. 84 (3): 283–292. doi:10.1016/j.jbspin.2016.06.012. PMID 27663756.
- ↑ Straino, S.; et al. (2004). "Enhanced arteriogenesis and wound repair in dystrophin-deficient mdx mice". Circulation. 110 (21): 3341–3348. doi: 10.1161/01.CIR.0000147776.50787.74 . PMID 15545520.
- ↑ Toprak, G.; et al. (2013). "Fibrosis in heart failure subtypes". Eur Rev Med Pharmacol Sci. 17 (17): 2302–9. PMID 24065222.
- ↑ Kamata, S.; et al. (2014). "Improvement of cardiac stem cell sheet therapy for chronic ischemic injury by adding endothelial progenitor cell transplantation: analysis of layer-specific regional cardiac function". Cell Transplant. 23 (10): 1305–19. doi:10.3727/096368913X665602. PMID 23562134. S2CID 25252653.
- ↑ Kapitsinou, P.P.; et al. (2014). "Endothelial HIF-2 mediates protection and recovery from ischemic kidney injury". J Clin Invest. 124 (6): 2396–2409. doi:10.1172/JCI69073. PMC 4092875 . PMID 24789906.
- ↑ van der Spoel TI (Sep 2011). "Human relevance of pre-clinical studies in stem cell therapy: systematic review and meta-analysis of large animal models of ischaemic heart disease". Cardiovasc Res. 91 (4): 649–58. doi: 10.1093/cvr/cvr113 . PMID 21498423.
- ↑ Zhao JJ (Jan 2014). "Protection of mesenchymal stem cells on acute kidney injury". Mol Med Rep. 9 (1): 91–96. doi: 10.3892/mmr.2013.1792 . PMID 24220681.
- ↑ Tan, X.; et al. (2016). "Angiopoietin-2 impairs collateral artery growth associated with the suppression of the infiltration of macrophages in mouse hindlimb ischemia". J Transl Med. 14 (1): 306. doi: 10.1186/s12967-016-1055-x . PMC 5080762 . PMID 27784306.
- ↑ Wu, J.; et al. (2015). "Plasminogen activator inhibitor-1 inhibits angiogenic signaling by uncoupling vascular endothelial growth factor receptor-2-alphaVbeta3 integrin cross talk". Arterioscler Thromb Vasc Biol. 35 (1): 111–20. doi:10.1161/ATVBAHA.114.304554. PMC 4270947 . PMID 25378411.
- ↑ Vaseva, A.V.; et al. (2012). "p53 opens the mitochondrial permeability transition pore to trigger necrosis". Cell. 149 (7): 1536–48. doi:10.1016/j.cell.2012.05.014. PMC 3383624 . PMID 22726440.
- ↑ Alam, M.R.; D. Baetz; M. Ovize (2015). "Cyclophilin D and myocardial ischemia-reperfusion injury: a fresh perspective". J Mol Cell Cardiol. 78: 80–9. doi:10.1016/j.yjmcc.2014.09.026. PMID 25281838.
- ↑ Pottecher, J.; et al. (2013). "Cyclosporine A normalizes mitochondrial coupling, reactive oxygen species production, and inflammation and partially restores skeletal muscle maximal oxidative capacity in experimental aortic cross-clamping". J Vasc Surg. 57 (4): 1100–1108 e2. doi: 10.1016/j.jvs.2012.09.020 . PMID 23332985.