
In geology and mineralogy, a mineral or mineral species is, broadly speaking, a solid chemical compound with a fairly well-defined chemical composition and a specific crystal structure that occurs naturally in pure form.

Silicon dioxide, also known as silica, is an oxide of silicon with the chemical formula SiO2, most commonly found in nature as quartz and in various living organisms. In many parts of the world, silica is the major constituent of sand. Silica is one of the most complex and most abundant families of materials, existing as a compound of several minerals and as a synthetic product. Notable examples include fused quartz, fumed silica, silica gel, opal and aerogels. It is used in structural materials, microelectronics, and as components in the food and pharmaceutical industries.

Shale is a fine-grained, clastic sedimentary rock formed from mud that is a mix of flakes of clay minerals and tiny fragments of other minerals, especially quartz and calcite. Shale is characterized by its tendency to split into thin layers (laminae) less than one centimeter in thickness. This property is called fissility. Shale is the most common sedimentary rock.

Bentonite is an absorbent swelling clay consisting mostly of montmorillonite which can either be Na-montmorillonite or Ca-montmorillonite. Na-montmorillonite has a considerably greater swelling capacity than Ca-montmorillonite.
The Bayer process is the principal industrial means of refining bauxite to produce alumina (aluminium oxide) and was developed by Carl Josef Bayer. Bauxite, the most important ore of aluminium, contains only 30–60% aluminium oxide (Al2O3), the rest being a mixture of silica, various iron oxides, and titanium dioxide. The aluminium oxide must be purified before it can be refined to aluminium metal.

Garnierite is a general name for a green nickel ore which is found in pockets and veins within weathered and serpentinized ultramafic rocks. It forms by lateritic weathering of ultramafic rocks and occurs in many nickel laterite deposits in the world. It is an important nickel ore, having a large weight percent NiO. As garnierite is not a valid mineral name according to the Commission on New Minerals, Nomenclature and Classification (CNMNC), no definite composition or formula has been universally adopted. Some of the proposed compositions are all hydrous Ni-Mg silicates, a general name for the Ni-Mg hydrosilicates which usually occur as an intimate mixture and commonly includes two or more of the following minerals: serpentine, talc, sepiolite, smectite, or chlorite, and Ni-Mg silicates, with or without alumina, that have x-ray diffraction patterns typical of serpentine, talc, sepiolite, chlorite, vermiculite or some mixture of them all.

Clay minerals are hydrous aluminium phyllosilicates, sometimes with variable amounts of iron, magnesium, alkali metals, alkaline earths, and other cations found on or near some planetary surfaces.

Silicate minerals are rock-forming minerals made up of silicate groups. They are the largest and most important class of minerals and make up approximately 90 percent of Earth's crust.

The chlorites are a group of phyllosilicate minerals common in low-grade metamorphic rocks and in altered igneous rocks. Greenschist, formed by metamorphism of basalt or other low-silica volcanic rock, typically contains significant amounts of chlorite.

Montmorillonite is a very soft phyllosilicate group of minerals that form when they precipitate from water solution as microscopic crystals, known as clay. It is named after Montmorillon in France. Montmorillonite, a member of the smectite group, is a 2:1 clay, meaning that it has two tetrahedral sheets of silica sandwiching a central octahedral sheet of alumina. The particles are plate-shaped with an average diameter around 1 μm and a thickness of 0.96 nm; magnification of about 25,000 times, using an electron microscope, is required to "see" individual clay particles. Members of this group include, amongst others, saponite, nontronite, beidellite, and hectorite.

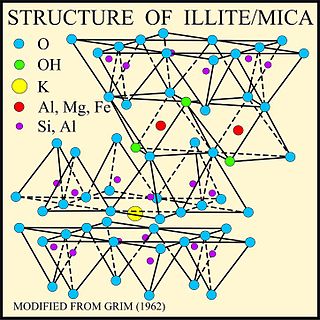
Illite is a group of closely related non-expanding clay minerals. Illite is a secondary mineral precipitate, and an example of a phyllosilicate, or layered alumino-silicate. Its structure is a 2:1 sandwich of silica tetrahedron (T) – alumina octahedron (O) – silica tetrahedron (T) layers. The space between this T-O-T sequence of layers is occupied by poorly hydrated potassium cations which are responsible for the absence of swelling. Structurally, illite is quite similar to muscovite with slightly more silicon, magnesium, iron, and water and slightly less tetrahedral aluminium and interlayer potassium. The chemical formula is given as (K,H3O)(Al,Mg,Fe)2(Si,Al)4O10[(OH)2·(H2O)], but there is considerable ion (isomorphic) substitution. It occurs as aggregates of small monoclinic grey to white crystals. Due to the small size, positive identification usually requires x-ray diffraction or SEM-EDS analysis. Illite occurs as an altered product of muscovite and feldspar in weathering and hydrothermal environments; it may be a component of sericite. It is common in sediments, soils, and argillaceous sedimentary rocks as well as in some low grade metamorphic rocks. The iron-rich member of the illite group, glauconite, in sediments can be differentiated by x-ray analysis.

A smectite is a mineral mixtures of various swelling sheet silicates (phyllosilicates), which have a three-layer 2:1 (TOT) structure and belong to the clay minerals. Smectites mainly consist of montmorillonite, but can often contain secondary minerals such as quartz and calcite.

Fault gouge is a tectonite with a very small grain size. Fault gouge has no cohesion and it is normally an unconsolidated rock type, unless cementation took place at a later stage. A fault gouge forms in the same way as fault breccia, the latter also having larger clasts. In comparison to fault breccia, which is another incohesive fault rock, fault gouge has a lower volume-fraction of visible fragments. Fault gouge is also classified as have particles that are smaller than 1mm in diameter. Therefore, fault gouge is commonly composed of clay minerals which commonly consist of illite, smectite, kaolinite, chlorite, sometimes vermiculite, along with unaltered detritic minerals produced by granite and gneiss erosion .. Here, TOT and TO respectively refer to the two more common layered crystal structures of clay minerals, where T represents the tetrahedral silica sheets and O the octahedral gibbsite sheets.

Iberulites are a particular type of microspherulites that develop in the atmosphere (troposphere), finally falling to the earth's surface. The name comes from the Iberian Peninsula where they were discovered.

Concrete degradation may have many different causes. Concrete is mostly damaged by the corrosion of reinforcement bars due to the carbonatation of hardened cement paste or chloride attack under wet conditions. Chemical damages are caused by the formation of expansive products produced by various chemical reactions, by aggressive chemical species present in groundwater and seawater, or by microorganisms. Other damaging processes can also involve calcium leaching by water infiltration and different physical phenomena initiating cracks formation and propagation. All these detrimental processes and damaging agents adversely affects the concrete mechanical strength and its durability.
The mineralogy of Mars is the chemical composition of rocks and soil that encompass the surface of Mars. Various orbital crafts have used spectroscopic methods to identify the signature of some minerals. The planetary landers performed concrete chemical analysis of the soil in rocks to further identify and confirm the presence of other minerals. The only samples of Martian rocks that are on Earth are in the form of meteorites. The elemental and atmospheric composition along with planetary conditions is essential in knowing what minerals can be formed from these base parts.

Rain dust or snow dust, traditionally known as muddy rain, red rain, or coloured rain, is a variety of rain which contains enough desert dust for the dust to be visible without using a microscope.
Illite crystallinity is a technique used to classify low-grade metamorphic activity in pelitic rocks. Determining the "illite crystallinity index" allows geologists to designate what metamorphic facies and metamorphic zone the rock was formed in and to infer what temperature the rock was formed. Several crystallinity indices have been proposed in recent years, but currently the Kübler index is being used due to its reproducibility and simplicity. The Kübler index is experimentally determined by measuring the full width at half maximum for the X-ray diffraction reflection peak along the (001) crystallographic axis of the rock sample. This value is an indirect measurement of the thickness of illite/muscovite packets which denote a change in metamorphic grade. The method can be used throughout the field of geology in areas such as the petroleum industry, plate tectonics.
In geology and mineralogy, a mineral group is a set of mineral species with essentially the same crystal structure and composed of chemically similar elements.
The soil matrix is the solid phase of soils, and comprise the solid particles that make up soils. Soil particles can be classified by their chemical composition (mineralogy) as well as their size. The particle size distribution of a soil, its texture, determines many of the properties of that soil, in particular hydraulic conductivity and water potential, but the mineralogy of those particles can strongly modify those properties. The mineralogy of the finest soil particles, clay, is especially important.
















