
In organic chemistry, an amide, also known as an organic amide or a carboxamide, is a compound with the general formula RC(=O)NR′R″, where R, R', and R″ represent organic groups or hydrogen atoms. The amide group is called a peptide bond when it is part of the main chain of a protein, and an isopeptide bond when it occurs in a side chain, such as in the amino acids asparagine and glutamine. It can be viewed as a derivative of a carboxylic acid with the hydroxyl group replaced by an amine group ; or, equivalently, an acyl (alkanoyl) group joined to an amine group.

Niacinamide or Nicotinamide (NAM) is a form of vitamin B3 found in food and used as a dietary supplement and medication. As a supplement, it is used by mouth to prevent and treat pellagra (niacin deficiency). While nicotinic acid (niacin) may be used for this purpose, niacinamide has the benefit of not causing skin flushing. As a cream, it is used to treat acne. It is a water-soluble vitamin. Niacinamide is the supplement name while Nicotinamide (NAM) is the scientific name.

A solvent (s) is a substance that dissolves a solute, resulting in a solution. A solvent is usually a liquid but can also be a solid, a gas, or a supercritical fluid. Water is a solvent for polar molecules and the most common solvent used by living things; all the ions and proteins in a cell are dissolved in water within the cell.
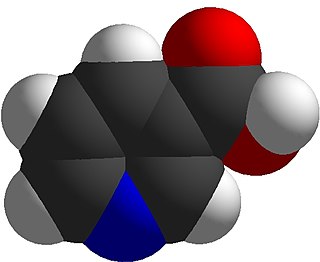
Niacin, also known as nicotinic acid, is an organic compound and a form of vitamin B3, an essential human nutrient. It can be manufactured by plants and animals from the amino acid tryptophan. Niacin is obtained in the diet from a variety of whole and processed foods, with highest contents in fortified packaged foods, meat, poultry, red fish such as tuna and salmon, lesser amounts in nuts, legumes and seeds. Niacin as a dietary supplement is used to treat pellagra, a disease caused by niacin deficiency. Signs and symptoms of pellagra include skin and mouth lesions, anemia, headaches, and tiredness. Many countries mandate its addition to wheat flour or other food grains, thereby reducing the risk of pellagra.

Valpromide is a carboxamide derivative of valproic acid used in the treatment of epilepsy and some affective disorders. It is rapidly metabolised (80%) to valproic acid but has anticonvulsant properties itself. It may produce more stable plasma levels than valproic acid or sodium valproate and may be more effective at preventing febrile seizures. However, it is over one hundred times more potent as an inhibitor of liver microsomal epoxide hydrolase. This makes it incompatible with carbamazepine and can affect the ability of the body to remove other toxins. Valpromide is no safer during pregnancy than valproic acid.

In organic chemistry, a carbodiimide is a functional group with the formula RN=C=NR. They are exclusively synthetic. A well known carbodiimide is dicyclohexylcarbodiimide, which is used in peptide synthesis. Dialkylcarbodiimides are stable. Some diaryl derivatives tend to convert to dimers and polymers upon standing at room temperature, though this mostly occurs with low melting point carbodiimides that are liquids at room temperature. Solid diaryl carbodiimides are more stable, but can slowly undergo hydrolysis in the presence of water over time.

Panthenol (also called pantothenol) is the alcohol analog of pantothenic acid (vitamin B5), and is thus a provitamin of B5. In organisms, it is quickly oxidized to pantothenic acid. It is a viscous transparent liquid at room temperature. Panthenol is used in pharmaceutical and cosmetic products as a moisturizer and to improve wound healing.

Di-tert-butyl dicarbonate is a reagent widely used in organic synthesis. Since this compound can be regarded formally as the acid anhydride derived from a tert-butoxycarbonyl (Boc) group, it is commonly referred to as Boc anhydride. This pyrocarbonate reacts with amines to give N-tert-butoxycarbonyl or so-called Boc derivatives. These carbamate derivatives do not behave as amines, which allows certain subsequent transformations to occur that would be incompatible with the amine functional group. The Boc group can later be removed from the amine using moderately strong acids. Thus, Boc serves as a protective group, for instance in solid phase peptide synthesis. Boc-protected amines are unreactive to most bases and nucleophiles, allowing for the use of the fluorenylmethyloxycarbonyl group (Fmoc) as an orthogonal protecting group.
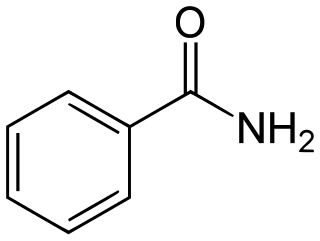
Benzamide is a organic compound with the chemical formula of C6H5C(O)NH2. It is the simplest amide derivative of benzoic acid. In powdered form, it appears as a white solid, while in cyrstalline form, it appears as colourless crystals. It is slightly soluble in water, and soluble in many organic solvents. It is a natural alkaloid found in the herbs of Berberis pruinosa.

Dimethyl carbonate (DMC) is an organic compound with the formula OC(OCH3)2. It is a colourless, flammable liquid. It is classified as a carbonate ester. This compound has found use as a methylating agent and more recently as a solvent that is exempt from the restrictions placed on most volatile organic compounds (VOCs) in the US. Dimethyl carbonate is often considered to be a green reagent.

The Erlenmeyer–Plöchl azlactone and amino acid synthesis, named after Friedrich Gustav Carl Emil Erlenmeyer who partly discovered the reaction, is a series of chemical reactions which transform an N-acyl glycine to various other amino acids via an oxazolone.
The molecular formula C6H6N2O may refer to:

Molsidomine is an orally active, long acting vasodilating drug used to treat angina pectoris. Molsidomine is metabolized in the liver to the active metabolite linsidomine. Linsidomine is an unstable compound that releases nitric oxide (NO) upon decay as the actual vasodilating compound.

Momordicin I, or 3,7,23-trihydroxycucurbitan-5,24-dien-19-al, is a chemical compound found in the leaves of the bitter melon vine, possibly responsible for its reputed medicinal properties.
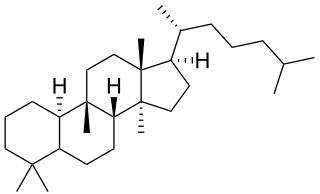
Cucurbitane is a class of chemical compounds with formula C
30H
54. It is a polycyclic hydrocarbon, specifically triterpene. It is also an isomer of lanostane, from which it differs by the formal shift of a methyl group from the 10 to the 9β position in the standard steroid numbering scheme.

Org 28611 (SCH-900,111) is a drug developed by Organon International which acts as a potent cannabinoid receptor full agonist at both the CB1 and CB2 receptors. It was developed with the aim of finding a water-soluble cannabinoid agonist suitable for intravenous use as an analgesic, and while it achieved this aim and has progressed as far as Phase II clinical trials in humans as both a sedative and an analgesic, results against the comparison drugs (midazolam and morphine respectively) were not particularly favourable in initial testing.
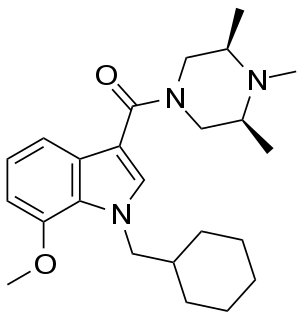
Org 28312 is a drug developed by Organon International which acts as a potent cannabinoid receptor full agonist at both the CB1 and CB2 receptors. It was developed with the aim of finding a water-soluble cannabinoid agonist suitable for intravenous use as an analgesic, but did not proceed to human trials, with the related compound Org 28611 chosen instead due to its better penetration into the brain. The structure-activity relationships of these compounds have subsequently been investigated further leading to the development of a number of more potent analogues, derived by cyclisation around the indole or piperazine rings.

Carbosulfan is an organic compound adherent to the carbamate class. At normal conditions, it is brown viscous liquid. It is not very stable; it decomposes slowly at room temperature. Its solubility in water is low but it is miscible with xylene, hexane, chloroform, dichloromethane, methanol and acetone. Carbosulfan is used as an insecticide. The European Union banned use of carbosulfan in 2007.
Hauser bases, also called magnesium amide bases, are magnesium compounds used in organic chemistry as bases for metalation reactions. These compounds were first described by Charles R. Hauser in 1947. Compared with organolithium reagents, the magnesium compounds have more covalent, and therefore less reactive, metal-ligand bonds. Consequently, they display a higher degree of functional group tolerance and a much greater chemoselectivity. Generally, Hauser bases are used at room temperature while reactions with organolithium reagents are performed at low temperatures, commonly at −78 °C.

The organic compound 3-pyridylnicotinamide (3-pna), also known as N-(pyridin-3-yl)nicotinamide, is a kinked dipodal dipyridine that is synthesized through the reaction of nicotinoyl chloride and 3-aminopyridine. The nitrogen atoms on its pyridine rings, like those of its isomer 4-pyridylnicotinamide, can donate their electron lone pairs to metal cations, allowing it to bridge metal centers and act as a bidentate ligand in coordination polymers. It can be used to synthesize polymers that have potentially useful gas adsorption properties.


















