Omega−3 fatty acids, also called omega−3 oils, ω−3 fatty acids or n−3 fatty acids, are polyunsaturated fatty acids (PUFAs) characterized by the presence of a double bond three atoms away from the terminal methyl group in their chemical structure. They are widely distributed in nature, are important constituents of animal lipid metabolism, and play an important role in the human diet and in human physiology. The three types of omega−3 fatty acids involved in human physiology are α-linolenic acid (ALA), eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA). ALA can be found in plants, while DHA and EPA are found in algae and fish. Marine algae and phytoplankton are primary sources of omega−3 fatty acids. DHA and EPA accumulate in fish that eat these algae. Common sources of plant oils containing ALA include walnuts, edible seeds, and flaxseeds as well as hempseed oil, while sources of EPA and DHA include fish and fish oils, and algae oil.

α-Linolenic acid, also known as alpha-linolenic acid (ALA), is an n−3, or omega-3, essential fatty acid. ALA is found in many seeds and oils, including flaxseed, walnuts, chia, hemp, and many common vegetable oils.

Arachidonic acid is a polyunsaturated omega−6 fatty acid 20:4(ω−6), or 20:4(5,8,11,14). If its precursors or diet contains linoleic acid it is formed by biosynthesis and can be deposited in animal fats. It is a precursor in the formation of leukotrienes, prostaglandins, and thromboxanes.

The pyrethrins are a class of organic compounds normally derived from Chrysanthemum cinerariifolium that have potent insecticidal activity by targeting the nervous systems of insects. Pyrethrin naturally occurs in chrysanthemum flowers and is often considered an organic insecticide when it is not combined with piperonyl butoxide or other synthetic adjuvants. Their insecticidal and insect-repellent properties have been known and used for thousands of years.
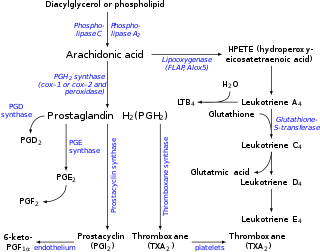
Eicosanoids are signaling molecules made by the enzymatic or non-enzymatic oxidation of arachidonic acid or other polyunsaturated fatty acids (PUFAs) that are, similar to arachidonic acid, around 20 carbon units in length. Eicosanoids are a sub-category of oxylipins, i.e. oxidized fatty acids of diverse carbon units in length, and are distinguished from other oxylipins by their overwhelming importance as cell signaling molecules. Eicosanoids function in diverse physiological systems and pathological processes such as: mounting or inhibiting inflammation, allergy, fever and other immune responses; regulating the abortion of pregnancy and normal childbirth; contributing to the perception of pain; regulating cell growth; controlling blood pressure; and modulating the regional flow of blood to tissues. In performing these roles, eicosanoids most often act as autocrine signaling agents to impact their cells of origin or as paracrine signaling agents to impact cells in the proximity of their cells of origin. Some eicosanoids, such as prostaglandins, may also have endocrine roles as hormones to influence the function of distant cells.

In organic chemistry, an allyl group is a substituent with the structural formula −CH2−HC=CH2. It consists of a methylene bridge attached to a vinyl group. The name is derived from the scientific name for garlic, Allium sativum. In 1844, Theodor Wertheim isolated an allyl derivative from garlic oil and named it "Schwefelallyl". The term allyl applies to many compounds related to H2C=CH−CH2, some of which are of practical or of everyday importance, for example, allyl chloride.
γ-Linolenic acid or GLA is an n−6, or omega-6, fatty acid found primarily in seed oils. When acting on GLA, arachidonate 5-lipoxygenase produces no leukotrienes and the conversion by the enzyme of arachidonic acid to leukotrienes is inhibited.
Linoleic acid (LA) is an organic compound with the formula HOOC(CH2)7CH=CHCH2CH=CH(CH2)4CH3. Both alkene groups are cis. It is a fatty acid sometimes denoted 18:2 (n−6) or 18:2 cis-9,12. A linoleate is a salt or ester of this acid.

Lipoxygenases (LOX) are a family of (non-heme) iron-containing enzymes, more specifically oxidative enzymes, most of which catalyze the dioxygenation of polyunsaturated fatty acids in lipids containing a cis,cis-1,4-pentadiene into cell signaling agents that serve diverse roles as autocrine signals that regulate the function of their parent cells, paracrine signals that regulate the function of nearby cells, and endocrine signals that regulate the function of distant cells.
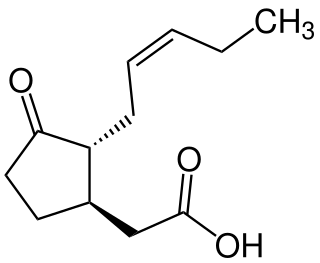
Jasmonic acid (JA) is an organic compound found in several plants including jasmine. The molecule is a member of the jasmonate class of plant hormones. It is biosynthesized from linolenic acid by the octadecanoid pathway. It was first isolated in 1957 as the methyl ester of jasmonic acid by the Swiss chemist Édouard Demole and his colleagues.
Pinolenic acid is a fatty acid contained in Siberian Pine nuts, Korean Pine nuts and the seeds and xylem of other pine (Pinus) species. The highest percentage of pinolenic acid is found in Siberian pine nuts and the oil produced from them.

The octadecanoid pathway is a biosynthetic pathway for the production of the phytohormone jasmonic acid (JA), an important hormone for induction of defense genes. JA is synthesized from alpha-linolenic acid, which can be released from the plasma membrane by certain lipase enzymes. For example, in the wound defense response, phospholipase C will cause the release of alpha-linolenic acid for JA synthesis.
In organic chemistry, pentadiene is any hydrocarbon with an open chain of five carbons, connected by two single bonds and two double bonds. All those compounds have the same molecular formula C5H8. The inventory of pentadienes include:

Chrysanthemic acid is an organic compound that is related to a variety of natural and synthetic insecticides. It is related to the pyrethrin I and II, as well as the pyrethroids. One of the four stereoisomers, (1R,3R)- or (+)-trans-chrysanthemic acid (pictured), is the acid part of the ester pyrethrin I, which occurs naturally in the seed cases of Chrysanthemum cinerariaefolium. Many synthetic pyrethroids, for example the allethrins, are esters of all four stereoisomers. Staudinger and Ružička named chrysanthemic acid in 1924.

Mead acid is an omega-9 fatty acid, first characterized by James F. Mead. As with some other omega-9 polyunsaturated fatty acids, animals can make Mead acid de novo. Its elevated presence in the blood is an indication of essential fatty acid deficiency. Mead acid is found in large quantities in cartilage.

ALOX15 is, like other lipoxygenases, a seminal enzyme in the metabolism of polyunsaturated fatty acids to a wide range of physiologically and pathologically important products. ▼ Gene Function

In enzymology, an allene-oxide cyclase is an enzyme that belongs to the family of isomerases, specifically a class of other intramolecular oxidoreductases. The systematic name of this enzyme class is (9Z)-(13S)-12,13-epoxyoctadeca-9,11,15-trienoate isomerase (cyclizing).

In enzymology, a chrysanthemyl diphosphate synthase is an enzyme involved in the biosynthesis of terpenoids. This enzyme is also known as CPPase. It catalyzes the chemical reaction shown below :
Poxytrins or dihydroxy-E,Z,E-polyunsaturated fatty acids (dihydroxy-E,Z,E-PUFAs) are PUFA metabolites that possess two hydroxyl residues and three in-series conjugated double bonds in an E,Z,E cis–trans configuration. Poxytrins have platelet-inhibiting properties that are not found in isomers with three conjugated double bonds presenting in a different geometry. The unique E,Z,E configuration in poxytrins may prove to be relevant in treating human conditions and diseases that involve pathological platelet activation.

In organic chemistry, an allene oxide is an epoxide of an allene. The parent allene oxide is CH2=C(O)CH2 (CAS RN 40079-14-9), a rare and reactive species of only theoretical interest. Typical allene oxides require steric protection for their isolation. Certain derivatives can be prepared by epoxidation of the allenes with peracetic acid. Allene oxides tend to rearrange to cyclopropanones.
















