
In geology and mineralogy, a mineral or mineral species is, broadly speaking, a solid substance with a fairly well-defined chemical composition and a specific crystal structure that occurs naturally in pure form.

Prehnite is an inosilicate of calcium and aluminium with the formula: Ca2Al(AlSi3O10)(OH)2 with limited Fe3+ substitutes for aluminium in the structure. Prehnite crystallizes in the orthorhombic crystal system, and most often forms as stalactitic, botryoidal, reniform or globular aggregates, with only just the crests of small crystals showing any faces, which are almost always curved or composite. Very rarely will it form distinct, well-individualized crystals showing a square-like cross-section, including those found at the Jeffrey Mine in Asbestos, Quebec, Canada. Prehnite is brittle with an uneven fracture and a vitreous to pearly luster. Its hardness is 6.5, its specific gravity is 2.80–2.95 and its color varies from light green to yellow, but also colorless, blue, pink or white. In April 2000, rare orange prehnite was discovered in the Kalahari Manganese Fields, South Africa. Prehnite is mostly translucent, and rarely transparent.

The pyroxenes are a group of important rock-forming inosilicate minerals found in many igneous and metamorphic rocks. Pyroxenes have the general formula XY(Si,Al)2O6, where X represents calcium (Ca), sodium (Na), iron or magnesium (Mg) and more rarely zinc, manganese or lithium, and Y represents ions of smaller size, such as chromium (Cr), aluminium (Al), magnesium (Mg), cobalt (Co), manganese (Mn), scandium (Sc), titanium (Ti), vanadium (V) or even iron. Although aluminium substitutes extensively for silicon in silicates such as feldspars and amphiboles, the substitution occurs only to a limited extent in most pyroxenes. They share a common structure consisting of single chains of silica tetrahedra. Pyroxenes that crystallize in the monoclinic system are known as clinopyroxenes and those that crystallize in the orthorhombic system are known as orthopyroxenes.

Epidote is a calcium aluminium iron sorosilicate mineral.

Vivianite (Fe2+
3(PO
4)
2·8H
2O) is a hydrated iron phosphate mineral found in a number of geological environments. Small amounts of manganese Mn2+, magnesium Mg2+, and calcium Ca2+ may substitute for iron Fe2+ in the structure. Pure vivianite is colorless, but the mineral oxidizes very easily, changing the color, and it is usually found as deep blue to deep bluish green prismatic to flattened crystals. Vivianite crystals are often found inside fossil shells, such as those of bivalves and gastropods, or attached to fossil bone. Vivianite can also appear on the iron coffins or on the corpses of humans as a result of a chemical reaction of the decomposing body with the iron enclosure.

Forsterite (Mg2SiO4; commonly abbreviated as Fo; also known as white olivine) is the magnesium-rich end-member of the olivine solid solution series. It is isomorphous with the iron-rich end-member, fayalite. Forsterite crystallizes in the orthorhombic system (space group Pbnm) with cell parameters a 4.75 Å (0.475 nm), b 10.20 Å (1.020 nm) and c 5.98 Å (0.598 nm).

Silicate minerals are rock-forming minerals made up of silicate groups. They are the largest and most important class of minerals and make up approximately 90 percent of Earth's crust.

Clinozoisite is a complex calcium aluminium sorosilicate mineral with formula: Ca2Al3(Si2O7)(SiO4)O(OH). It forms a continuous solid solution series with epidote by substitution of iron(III) in the aluminium (m3 site) and is also called aluminium epidote.

Vauxite is a phosphate mineral with the chemical formula Fe2+Al2(PO4)2(OH)2·6(H2O). It belongs to the laueite – paravauxite group, paravauxite subgroup, although Mindat puts it as a member of the vantasselite Al4(PO4)3(OH)3·9H2O group. There is no similarity in structure between vauxite and paravauxite Fe2+Al2(PO4)2(OH)2·8H2O or metavauxite Fe3+Al2(PO4)2(OH)2·8H2O, even though they are closely similar chemically and all minerals occur together as secondary minerals. Vauxite was named in 1922 for George Vaux Junior (1863–1927), an American attorney and mineral collector.

Pumpellyite is a group of closely related sorosilicate minerals:
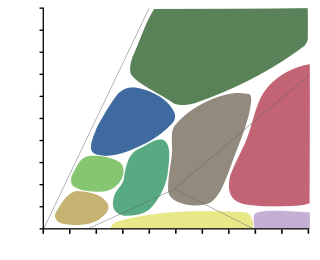
A metamorphic facies is a set of mineral assemblages in metamorphic rocks formed under similar pressures and temperatures. The assemblage is typical of what is formed in conditions corresponding to an area on the two dimensional graph of temperature vs. pressure. Rocks which contain certain minerals can therefore be linked to certain tectonic settings, times and places in the geological history of the area. The boundaries between facies are wide because they are gradational and approximate. The area on the graph corresponding to rock formation at the lowest values of temperature and pressure is the range of formation of sedimentary rocks, as opposed to metamorphic rocks, in a process called diagenesis.

Piemontite is a sorosilicate mineral in the monoclinic crystal system with the chemical formula Ca2(Al,Mn3+,Fe3+)3(SiO4)(Si2O7)O(OH). It is a member of the epidote group.

Dollaseite-(Ce) is a sorosilicate end-member epidote rare-earth mineral which was discovered by Per Geijer (1927) in the Ostanmossa mine, Norberg district, Sweden. Dollaseite-(Ce), although not very well known, is part of a broad epidote group of minerals which are primarily silicates, the most abundant type of minerals on earth. Dollaseite-(Ce) forms as dark-brown subhedral crystals primarily in Swedish mines. With the ideal chemical formula, CaREE3+
Mg
2AlSi
3O
11,(OH)F, dollaseite-(Ce) can be partially identified by its content of the rare earth element cerium.

Beraunite is an iron phosphate mineral. It was first described by August Breithaupt for an occurrence in Beraun currently in the Czech Republic. Beraunite occurs as a secondary mineral in iron ore deposits, and as an alteration product of primary phosphate minerals in granite pegmatites.
This list gives an overview of the classification of minerals (silicates) and includes mostly International Mineralogical Association (IMA) recognized minerals and its groupings. This list complements the List of minerals recognized by the International Mineralogical Association series of articles and List of minerals. Rocks, ores, mineral mixtures, non-IMA approved minerals and non-named minerals are mostly excluded.

Tuperssuatsiaite is a rare clay mineral found in Greenland, Namibia and Brazil. It is a hydrated phyllosilicate of sodium and iron.

Gugiaite is a melilite mineral, named for the Chinese village of Gugia where it was first discovered. Its chemical formula is Ca2BeSi2O7. It occurs mostly in skarns with melanite adjacent to an alkali syenite and has no economic value. Its crystals are small tetragonal tablets with vitreous luster and perfect cleavage. It is colorless and transparent with a density of three. The mineral belongs to space group P421m and is strongly piezoelectric.

A subduction zone is a region of the Earth's crust where one tectonic plate moves under another tectonic plate; oceanic crust gets recycled back into the mantle and continental crust gets produced by the formation of arc magmas. Arc magmas account for more than 20% of terrestrially produced magmas and are produced by the dehydration of minerals within the subducting slab as it descends into the mantle and are accreted onto the base of the overriding continental plate. Subduction zones host a unique variety of rock types formed by the high-pressure, low-temperature conditions a subducting slab encounters during its descent. The metamorphic conditions the slab passes through in this process generates and alters water bearing (hydrous) mineral phases, releasing water into the mantle. This water lowers the melting point of mantle rock, initiating melting. Understanding the timing and conditions in which these dehydration reactions occur, is key to interpreting mantle melting, volcanic arc magmatism, and the formation of continental crust.
In geology and mineralogy, a mineral group is a set of mineral species with essentially the same crystal structure and composed of chemically similar elements.

















