
Vanadium is a chemical element; it has symbol V and atomic number 23. It is a hard, silvery-grey, malleable transition metal. The elemental metal is rarely found in nature, but once isolated artificially, the formation of an oxide layer (passivation) somewhat stabilizes the free metal against further oxidation.

Vanadinite is a mineral belonging to the apatite group of phosphates, with the chemical formula Pb5(VO4)3Cl. It is one of the main industrial ores of the metal vanadium and a minor source of lead. A dense, brittle mineral, it is usually found in the form of red hexagonal crystals. It is an uncommon mineral, formed by the oxidation of lead ore deposits such as galena. First discovered in 1801 in Mexico, vanadinite deposits have since been unearthed in South America, Europe, Africa, and North America.

Carnotite is a potassium uranium vanadate radioactive mineral with chemical formula K2(UO2)2(VO4)2·3H2O. The water content can vary and small amounts of calcium, barium, magnesium, iron, and sodium are often present.

Group 5 is a group of elements in the periodic table. Group 5 contains vanadium (V), niobium (Nb), tantalum (Ta) and dubnium (Db). This group lies in the d-block of the periodic table. This group is sometimes called the vanadium group or vanadium family after its lightest member; however, the group itself has not acquired a trivial name because it belongs to the broader grouping of the transition metals.
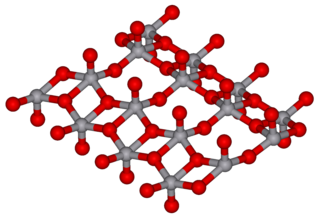
Vanadium(V) oxide (vanadia) is the inorganic compound with the formula V2O5. Commonly known as vanadium pentoxide, it is a brown/yellow solid, although when freshly precipitated from aqueous solution, its colour is deep orange. Because of its high oxidation state, it is both an amphoteric oxide and an oxidizing agent. From the industrial perspective, it is the most important compound of vanadium, being the principal precursor to alloys of vanadium and is a widely used industrial catalyst.

Cavansite, whose name is derived from its chemical composition, calcium vanadium silicate, is a deep blue hydrous calcium vanadium phyllosilicate mineral, occurring as a secondary mineral in basaltic and andesitic rocks along with a variety of zeolite minerals. Its blue coloring comes from vanadium, a metal ion. Discovered in 1967 in Malheur County, Oregon, cavansite is a relatively rare mineral. It is polymorphic with the even rarer mineral, pentagonite. It is most frequently found in Pune, India, and in the Deccan Traps, a large igneous province.
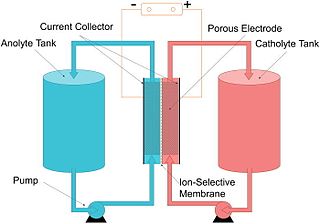
The vanadium redox battery (VRB), also known as the vanadium flow battery (VFB) or vanadium redox flow battery (VRFB), is a type of rechargeable flow battery. It employs vanadium ions as charge carriers. The battery uses vanadium's ability to exist in a solution in four different oxidation states to make a battery with a single electroactive element instead of two. For several reasons, including their relative bulkiness, vanadium batteries are typically used for grid energy storage, i.e., attached to power plants/electrical grids.

Descloizite is a rare mineral species consisting of basic lead and zinc vanadate, (Pb, Zn)2(OH)VO4, crystallizing in the orthorhombic crystal system and isomorphous with olivenite. Appreciable gallium and germanium may also be incorporated into the crystal structure.

Sodium metavanadate is the inorganic compound with the formula NaVO3. It is a yellow, water-soluble salt.

Vanadium(III) oxide is the inorganic compound with the formula V2O3. It is a black solid prepared by reduction of V2O5 with hydrogen or carbon monoxide. It is a basic oxide dissolving in acids to give solutions of vanadium (III) complexes. V2O3 has the corundum structure. It is antiferromagnetic with a critical temperature of 160 K. At this temperature there is an abrupt change in conductivity from metallic to insulating. This also distorts the crystal structure to a monoclinic space group: C2/c.

Vanadium(II) oxide is the inorganic compound with the idealized formula VO. It is one of the several binary vanadium oxides. It adopts a distorted NaCl structure and contains weak V−V metal to metal bonds. VO is a semiconductor owing to delocalisation of electrons in the t2g orbitals. VO is a non-stoichiometric compound, its composition varying from VO0.8 to VO1.3.

Vanadyl(IV) sulfate describes a collection of inorganic compounds of vanadium with the formula, VOSO4(H2O)x where 0 ≤ x ≤ 6. The pentahydrate is common. This hygroscopic blue solid is one of the most common sources of vanadium in the laboratory, reflecting its high stability. It features the vanadyl ion, VO2+, which has been called the "most stable diatomic ion".
Vanadium oxide may refer to:

Uranium mining in Colorado, United States, goes back to 1872, when pitchblende ore was taken from gold mines near Central City, Colorado. The Colorado uranium industry has seen booms and busts, but continues to this day. Not counting byproduct uranium from phosphate, Colorado is considered to have the third largest uranium reserves of any US state, behind Wyoming and New Mexico.

Roscoelite is a green mineral from the mica group that contains vanadium.

Uranium mining in Utah, a state of the United States, has a history going back more than 100 years. Uranium mining started as a byproduct of vanadium mining about 1900, became a byproduct of radium mining about 1910, then back to a byproduct of vanadium when the radium price fell in the 1920s. Utah saw a uranium boom in the late 1940s and early 1950s, but uranium mining declined in the 1980s. Since 2001 there has been a revival of interest in uranium mining, as a result of higher uranium prices.

Patrónite is the vanadium sulfide mineral with formula VS4. The material is usually described as V4+(S22−)2. Structurally, it is a "linear-chain" compound with alternating bonding and nonbonding contacts between the vanadium centers. The vanadium is octa-coordinated, which is an uncommon geometry for this metal.

Conichalcite, CaCu(AsO4)(OH), is a relatively common arsenate mineral related to duftite (PbCu(AsO4)(OH)). It is green, often botryoidal, and occurs in the oxidation zone of some metal deposits. It occurs with limonite, malachite, beudantite, adamite, cuproadamite, olivenite and smithsonite.
Colimaite, the naturally occurring analog of synthetic K3VS4, is a sulfide mineral discovered in southwestern Mexico. The potassium-vanadium sulfide was collected from the crater of the Colima volcano. The mineral colimaite is named after the locality of this volcano and has been approved in 2007, along with its mineral name, by the Commission on New Minerals, Nomenclature and Classification (CNMNC). It has been given the International Mineralogical Association number of IMA 2007–045.

The global vanadium cycle is controlled by physical and chemical processes that drive the exchange of vanadium between its two main reservoirs: the upper continental crust and the ocean. Anthropogenic processes such as coal and petroleum production release vanadium to the atmosphere.

















