
Lamins, also known as nuclear lamins are fibrous proteins in type V intermediate filaments, providing structural function and transcriptional regulation in the cell nucleus. Nuclear lamins interact with inner nuclear membrane proteins to form the nuclear lamina on the interior of the nuclear envelope. Lamins have elastic and mechanosensitive properties, and can alter gene regulation in a feedback response to mechanical cues. Lamins are present in all animals but are not found in microorganisms, plants or fungi. Lamin proteins are involved in the disassembling and reforming of the nuclear envelope during mitosis, the positioning of nuclear pores, and programmed cell death. Mutations in lamin genes can result in several genetic laminopathies, which may be life-threatening.

The nuclear lamina is a dense fibrillar network inside the nucleus of most cells. It is composed of intermediate filaments and membrane associated proteins. Besides providing mechanical support, the nuclear lamina regulates important cellular events such as DNA replication and cell division. Additionally, it participates in chromatin organization and it anchors the nuclear pore complexes embedded in the nuclear envelope.

Emerin is a protein that in humans is encoded by the EMD gene, also known as the STA gene. Emerin, together with LEMD3, is a LEM domain-containing integral protein of the inner nuclear membrane in vertebrates. Emerin is highly expressed in cardiac and skeletal muscle. In cardiac muscle, emerin localizes to adherens junctions within intercalated discs where it appears to function in mechanotransduction of cellular strain and in beta-catenin signaling. Mutations in emerin cause X-linked recessive Emery–Dreifuss muscular dystrophy, cardiac conduction abnormalities and dilated cardiomyopathy.
Lamina-associated polypeptide 2 (LAP2), isoforms beta/gamma is a protein that in humans is encoded by the TMPO gene. LAP2 is an inner nuclear membrane (INM) protein.

Laminopathies are a group of rare genetic disorders caused by mutations in genes encoding proteins of the nuclear lamina. They are included in the more generic term nuclear envelopathies that was coined in 2000 for diseases associated with defects of the nuclear envelope. Since the first reports of laminopathies in the late 1990s, increased research efforts have started to uncover the vital role of nuclear envelope proteins in cell and tissue integrity in animals.

LMNA, also known as Lamin A/C is a protein that in humans is encoded by the LMNA gene. Lamin A/C belongs to the lamin family of proteins.

Lamin-B receptor is a protein, and in humans, it is encoded by the LBR gene.

Chromobox protein homolog 3 is a protein that is encoded by the CBX3 gene in humans.

Nuclear pore glycoprotein-210 (gp210) is an essential trafficking regulator in the eukaryotic nuclear pore complex. Gp-210 anchors the pore complex to the nuclear membrane. and protein tagging reveals its primarily located on the luminal side of double layer membrane at the pore. A single polypeptide motif of gp210 is responsible for sorting to nuclear membrane, and indicate the carboxyl tail of the protein is oriented toward the cytoplasmic side of the membrane.

The nuclear envelope, also known as the nuclear membrane, is made up of two lipid bilayer membranes which in eukaryotic cells surrounds the nucleus, which encases the genetic material.

Buschke–Ollendorff syndrome is a rare genetic disorder associated with LEMD3. It is believed to be inherited in an autosomal dominant manner. It is named for Abraham Buschke and Helene Ollendorff Curth, who described it in a 45-year-old woman. Its frequency is almost 1 case per every 20,000 people, and it is equally found in both males and females.

Barrier-to-autointegration factor is a protein that in humans is encoded by the BANF1 gene. It is a member of the barrier-to-autointegration factor family of proteins.

PAK3 is one of three members of Group I PAK family of evolutionary conserved serine/threonine kinases. PAK3 is preferentially expressed in neuronal cells and involved in synapse formation and plasticity and mental retardation.

POU domain, class 3, transcription factor 1 is a protein that in humans is encoded by the POU3F1 gene.

Cytochrome c oxidase subunit 4 isoform 2, mitochondrial is an enzyme that in humans is encoded by the COX4I2 gene. COX4I2 is a nuclear-encoded isoform of cytochrome c oxidase (COX) subunit 4. Cytochrome c oxidase is a multi-subunit enzyme complex that couples the transfer of electrons from cytochrome c to molecular oxygen and contributes to a proton electrochemical gradient across the inner mitochondrial membrane, acting as the terminal enzyme of the mitochondrial respiratory chain. Mutations in COX4I2 have been associated with exocrine pancreatic insufficiency, dyserythropoietic anemia, and calvarial hyperostosis (EPIDACH).

Torsin-1A-interacting protein 1 is a protein that in humans is encoded by the TOR1AIP1 gene. More commonly known as lamina associated polypeptide 1 (LAP1), it is a type II integral membrane protein that resides in the inner nuclear membrane. The luminal domain of LAP1 interacts with Torsin A and is necessary for the ATPase activity of Torsin A. LAP1 plays a critical role in skeletal and heart muscle. Mutations in TOR1AIP1 have been linked to muscular dystrophy and cardiomyopathy. It's deletion from mouse hepatocytes leads to defected very-low density lipoprotein secretion and causes non-alcoholic fatty liver disease and non-alcoholic steatohepatitis

Nuclear prelamin A recognition factor, also known as NARF, is a protein which in humans is encoded by the NARF gene.

Lamin-B1 is a protein that in humans is encoded by the LMNB1 gene.
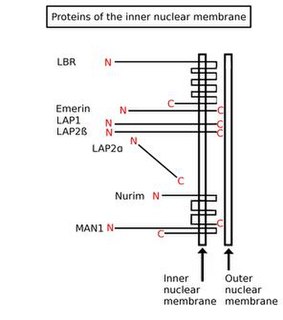
Inner nuclear membrane (INM) proteins are proteins that are embedded in or associated with the inner membrane of the nuclear envelope (NE). There are about 60 INM proteins, most of which are poorly characterized with respect to structure and function. Among the few well-characterized INM proteins are lamin B receptor (LBR), lamina-associated polypeptide 1 (LAP1), lamina-associated polypeptide-2 (LAP2), emerin and MAN1.
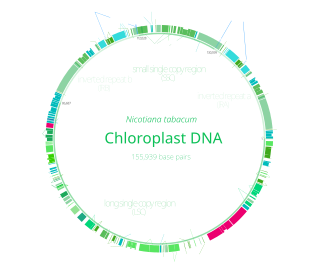
Chloroplasts have their own DNA, often abbreviated as cpDNA. It is also known as the plastome when referring to genomes of other plastids. Its existence was first proven in 1962. The first complete chloroplast genome sequences were published in 1986, Nicotiana tabacum (tobacco) by Sugiura and colleagues and Marchantia polymorpha (liverwort) by Ozeki et al. Since then, hundreds of chloroplast DNAs from various species have been sequenced, but they are mostly those of land plants and green algae—glaucophytes, red algae, and other algae groups are extremely underrepresented, potentially introducing some bias in views of "typical" chloroplast DNA structure and content.




















