
A detergent is a surfactant or a mixture of surfactants with cleansing properties when in dilute solutions. There are a large variety of detergents, a common family being the alkylbenzene sulfonates, which are soap-like compounds that are more soluble in hard water, because the polar sulfonate is less likely than the polar carboxylate to bind to calcium and other ions found in hard water.

Surfactants are chemical compounds that decrease the surface tension or interfacial tension between two liquids, a liquid and a gas, or a liquid and a solid. Surfactants may function as emulsifiers, wetting agents, detergents, foaming agents, or dispersants. The word "surfactant" is a blend of surface-active agent, coined c. 1950.
A lysis buffer is a buffer solution used for the purpose of breaking open cells for use in molecular biology experiments that analyze the labile macromolecules of the cells. Most lysis buffers contain buffering salts and ionic salts to regulate the pH and osmolarity of the lysate. Sometimes detergents are added to break up membrane structures. For lysis buffers targeted at protein extraction, protease inhibitors are often included, and in difficult cases may be almost required. Lysis buffers can be used on both animal and plant tissue cells.

Sodium triphosphate (STP), also sodium tripolyphosphate (STPP), or tripolyphosphate (TPP),) is an inorganic compound with formula Na5P3O10. It is the sodium salt of the polyphosphate penta-anion, which is the conjugate base of triphosphoric acid. It is produced on a large scale as a component of many domestic and industrial products, especially detergents. Environmental problems associated with eutrophication are attributed to its widespread use.
In organic chemistry, ethoxylation is a chemical reaction in which ethylene oxide adds to a substrate. It is the most widely practiced alkoxylation, which involves the addition of epoxides to substrates.

In organic chemistry, sulfonic acid refers to a member of the class of organosulfur compounds with the general formula R−S(=O)2−OH, where R is an organic alkyl or aryl group and the S(=O)2(OH) group a sulfonyl hydroxide. As a substituent, it is known as a sulfo group. A sulfonic acid can be thought of as sulfuric acid with one hydroxyl group replaced by an organic substituent. The parent compound is the parent sulfonic acid, HS(=O)2(OH), a tautomer of sulfurous acid, S(=O)(OH)2. Salts or esters of sulfonic acids are called sulfonates.

Laundry detergent is a type of detergent used for cleaning dirty laundry (clothes). Laundry detergent is manufactured in powder and liquid form.

An amphiphile, or amphipath, is a chemical compound possessing both hydrophilic and lipophilic (fat-loving) properties. Such a compound is called amphiphilic or amphipathic. Amphiphilic compounds include surfactants. The phospholipid amphiphiles are the major structural component of cell membranes.
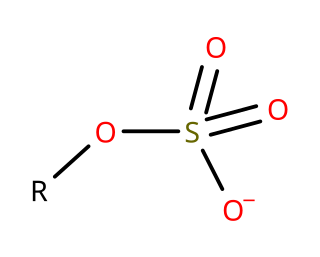
In organosulfur chemistry, organosulfates are a class of organic compounds sharing a common functional group with the structure R−O−SO−3. The SO4 core is a sulfate group and the R group is any organic residue. All organosulfates are formally esters derived from alcohols and sulfuric acid although many are not prepared in this way. Many sulfate esters are used in detergents, and some are useful reagents. Alkyl sulfates consist of a hydrophobic hydrocarbon chain, a polar sulfate group and either a cation or amine to neutralize the sulfate group. Examples include: sodium lauryl sulfate and related potassium and ammonium salts.
Dodecylbenzene is an organic compound with the formula C
12H
25C
6H
5. Dodecylbenzene is a colorless liquid with a weak oily odor that floats on water.

Cleaning agents or hard-surface cleaners are substances used to remove dirt, including dust, stains, foul odors, and clutter on surfaces. Purposes of cleaning agents include health, beauty, removing offensive odor, and avoiding the spread of dirt and contaminants to oneself and others. Some cleaning agents can kill bacteria and clean at the same time. Others, called degreasers, contain organic solvents to help dissolve oils and fats.

Lauryldimethylamine oxide (LDAO), also known as dodecyldimethylamine oxide (DDAO), is an amine oxide–based nonionic surfactant, with a C12 (dodecyl) alkyl tail. It is one of the most frequently-used surfactants of this type. Like other amine oxide–based surfactants it is antimicrobial, being effective against common bacteria such as S. aureus and E. coli, however, it is also non-denaturing and may be used to solubilize proteins.
A soap substitute is a natural or synthetic cleaning product used in place of soap or other detergents, typically to reduce environmental impact or health harms or provide other benefits.
A methylene blue active substances assay, or MBAS assay, is a colorimetric analysis test method that uses methylene blue to detect the presence of anionic surfactants in a sample of water. An anionic surfactant detected by the color reaction is called a methylene blue active substance (MBAS).
Paint has four major components: pigments, binders, solvents, and additives. Pigments serve to give paint its color, texture, toughness, as well as determining if a paint is opaque or not. Common white pigments include titanium dioxide and zinc oxide. Binders are the film forming component of a paint as it dries and affects the durability, gloss, and flexibility of the coating. Polyurethanes, polyesters, and acrylics are all examples of common binders. The solvent is the medium in which all other components of the paint are dissolved and evaporates away as the paint dries and cures. The solvent also modifies the curing rate and viscosity of the paint in its liquid state. There are two types of paint: solvent-borne and water-borne paints. Solvent-borne paints use organic solvents as the primary vehicle carrying the solid components in a paint formulation, whereas water-borne paints use water as the continuous medium. The additives that are incorporated into paints are a wide range of things which impart important effects on the properties of the paint and the final coating. Common paint additives are catalysts, thickeners, stabilizers, emulsifiers, texturizers, biocides to fight bacterial growth, etc.

An alkylbenzene is a chemical compound that contains a monocyclic aromatic ring attaching to one or more saturated hydrocarbon chains. Alkylbenzenes are derivatives of benzene, in which one or more hydrogen atoms are replaced by alkyl groups. The simplest member, toluene, has the hydrogen atom of the benzene ring replaced by a methyl group. The chemical formula of alkylbenzenes is CnH2n-6.
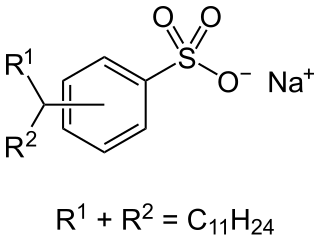
Alkylbenzene sulfonates are a class of anionic surfactants, consisting of a hydrophilic sulfonate head-group and a hydrophobic alkylbenzene tail-group. Along with sodium laureth sulfate, they are one of the oldest and most widely used synthetic detergents and may be found in numerous personal-care products and household-care products . They were introduced in the 1930s in the form of branched alkylbenzene sulfonates (BAS). However following environmental concerns these were replaced with linear alkylbenzene sulfonates (LAS) during the 1960s. Since then production has increased significantly from about one million tons in 1980, to around 3.5 million tons in 2016, making them most produced anionic surfactant after soaps.
Phosphates in detergent refers to the use of phosphates as an ingredient in a detergent product. The advantage of using phosphates in a consumer laundry detergent or dishwashing detergent is that they make detergents more efficient by chelating calcium and magnesium ions. The disadvantage of using phosphates is that they remain in wastewater and eventually make their way to a natural body of water. While phosphates are low toxicity, they instead cause nutrient pollution and feed the algae. This leads to eutrophication and harmful algal bloom.

Polycarboxylates are linear polymers with a high molecular mass and with many carboxylate groups. They are polymers of acrylic acid or copolymers of acrylic acid and maleic acid. The polymer is used as the sodium salt.
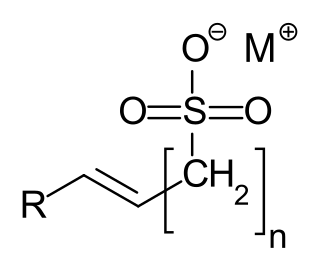
α-Olefin sulfonates are a group of anionic surfactants, which are used as detergents. The compounds contain a - mostly linear, primary - alkyl R and a monovalent cation M, preferably sodium. The most frequently used example of this group of substances is sodium α-olefin sulfonate.
















