
An HIV vaccine is a potential vaccine that could be either a preventive vaccine or a therapeutic vaccine, which means it would either protect individuals from being infected with HIV or treat HIV-infected individuals.
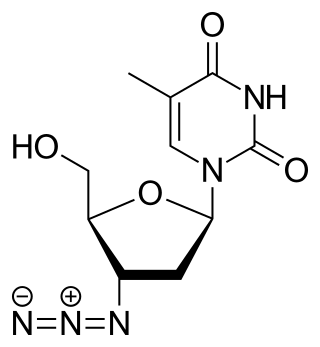
Zidovudine (ZDV), also known as azidothymidine (AZT), is an antiretroviral medication used to prevent and treat HIV/AIDS. It is generally recommended for use in combination with other antiretrovirals. It may be used to prevent mother-to-child spread during birth or after a needlestick injury or other potential exposure. It is sold both by itself and together as lamivudine/zidovudine and abacavir/lamivudine/zidovudine. It can be used by mouth or by slow injection into a vein.
The management of HIV/AIDS normally includes the use of multiple antiretroviral drugs as a strategy to control HIV infection. There are several classes of antiretroviral agents that act on different stages of the HIV life-cycle. The use of multiple drugs that act on different viral targets is known as highly active antiretroviral therapy (HAART). HAART decreases the patient's total burden of HIV, maintains function of the immune system, and prevents opportunistic infections that often lead to death. HAART also prevents the transmission of HIV between serodiscordant same-sex and opposite-sex partners so long as the HIV-positive partner maintains an undetectable viral load.

Didanosine, sold under the brand name Videx, is a medication used to treat HIV/AIDS. It is used in combination with other medications as part of highly active antiretroviral therapy (HAART). It is of the reverse-transcriptase inhibitor class.
Post-exposure prophylaxis, also known as post-exposure prevention (PEP), is any preventive medical treatment started after exposure to a pathogen in order to prevent the infection from occurring.

Nevirapine (NVP), sold under the brand name Viramune among others, is a medication used to treat and prevent HIV/AIDS, specifically HIV-1. It is generally recommended for use with other antiretroviral medications. It may be used to prevent mother to child spread during birth but is not recommended following other exposures. It is taken by mouth.

The Wistar Institute is an independent, nonprofit research institution in biomedical science with special focuses in oncology, immunology, infectious disease and vaccine research. Located on Spruce Street in Philadelphia’s University City neighborhood, Wistar was founded in 1892 as a nonprofit institution to focus on biomedical research and training.

Infection with HIV, a retrovirus, can be managed with treatment but without treatment can lead to a spectrum of conditions including AIDS.
Long-term nonprogressors (LTNPs), sometimes also called elite controllers, are individuals infected with HIV, who maintain a CD4 count greater than 500 without antiretroviral therapy with a detectable viral load. Many of these patients have been HIV positive for 30 years without progressing to the point of needing to take medication in order not to develop AIDS. They have been the subject of a great deal of research, since an understanding of their ability to control HIV infection may lead to the development of immune therapies or a therapeutic vaccine. The classification "Long-term non-progressor" is not permanent, because some patients in this category have gone on to develop AIDS.

HIV/AIDS research includes all medical research that attempts to prevent, treat, or cure HIV/AIDS, as well as fundamental research about the nature of HIV as an infectious agent and AIDS as the disease caused by HIV.
The co-epidemic of tuberculosis (TB) and human immunodeficiency virus (HIV) is one of the major global health challenges in the present time. The World Health Organization (WHO) reports 9.2 million new cases of TB in 2006 of whom 7.7% were HIV-infected. Tuberculosis is the most common contagious infection in HIV-Immunocompromised patients leading to death. These diseases act in combination as HIV drives a decline in immunity while tuberculosis progresses due to defective immune status. This condition becomes more severe in case of multi-drug (MDRTB) and extensively drug resistant TB (XDRTB), which are difficult to treat and contribute to increased mortality. Tuberculosis can occur at any stage of HIV infection. The risk and severity of tuberculosis increases soon after infection with HIV. A study on gold miners of South Africa revealed that the risk of TB was doubled during the first year after HIV seroconversion. Although tuberculosis can be a relatively early manifestation of HIV infection, it is important to note that the risk of tuberculosis progresses as the CD4 cell count decreases along with the progression of HIV infection. The risk of TB generally remains high in HIV-infected patients, remaining above the background risk of the general population even with effective immune reconstitution and high CD4 cell counts with antiretroviral therapy.
Vaccinia immune globulin (VIG) is made from the pooled blood of individuals who have been inoculated with the smallpox vaccine. The antibodies these individuals developed in response to the smallpox vaccine are removed and purified. This results in VIG. It can be administered intravenously. It is used to treat individuals who have developed progressive vaccinia after smallpox vaccination.
Deborah Persaud is a Guyanese-born American virologist who primarily works on HIV/AIDS at Johns Hopkins Children's Center.
HIV in pregnancy is the presence of an HIV/AIDS infection in a woman while she is pregnant. There is a risk of HIV transmission from mother to child in three primary situations: pregnancy, childbirth, and while breastfeeding. This topic is important because the risk of viral transmission can be significantly reduced with appropriate medical intervention, and without treatment HIV/AIDS can cause significant illness and death in both the mother and child. This is exemplified by data from The Centers for Disease Control (CDC): In the United States and Puerto Rico between the years of 2014–2017, where prenatal care is generally accessible, there were 10,257 infants in the United States and Puerto Rico who were exposed to a maternal HIV infection in utero who did not become infected and 244 exposed infants who did become infected.
Julianna Lisziewicz is a Hungarian immunologist. Lisziewicz headed many research teams that have discovered and produced immunotheraputic drugs to treat diseases like cancer and chronic infections like HIV/AIDS. Some of these drugs have been successfully used in clinical trials.

Nadia Lauren Dowshen is an American pediatrician and adolescent medicine physician. She specializes in the care of youth living with HIV infection and medical care to transgender and gender-diverse youth. Dowshen researches health inequality, access to care, and promoting resilience in LGBT youth. As an associate professor at the Perelman School of Medicine at the University of Pennsylvania, she is also the medical director and co-founder of the Gender and Sexuality Development Clinic.
Crystal C. Watkins Johansson is an American neuroscientist and psychiatrist and associate professor of neuroscience at Johns Hopkins University School of Medicine as well as the director of the Sheppard Pratt Memory Clinic in Neuropsychiatry in Baltimore, Maryland. Johansson was the first Black female Meyerhoff Scholar to obtain an MD/PhD from the University of Maryland, Baltimore County. During her MD/PhD she developed a novel treatment for gastrointestinal in patients with diabetes that led to a patent for a pharmacological compound in 2000. Johansson is a practicing neuropsychiatrist with a focus on geriatric psychiatry and she conducts brain imaging research as well as research on cancer in African American women.

Andrew Ddungu Kambugu is a Ugandan physician who serves as The Sande-McKinnell Executive Director at Uganda Infectious Disease Institute and a Honorary Senior lecturer at Makerere University College of Sciences. He is also an Adjunct Associate Professor at the University of Minnesota. In July 2020, he was appointed to the United Nations 2021 Food System Scientific Group.

Nirali N. Shah is an American physician-scientist and pediatric hematologist-oncologist, serving as head of the hematologic malignancies section of the pediatric oncology branch at the National Cancer Institute. She researches the translation of immunotherapeutic approaches to treat high-risk hematologic malignancies in children, adolescents and young adults.

Judith Aberg is an American physician who is the George Baehr Professor of Clinical Medicine at Mount Sinai Hospital. She was appointed Dean of System Operations for Clinical Sciences at the Icahn School of Medicine at Mount Sinai. Her research considered infectious diseases, including HIV/AIDS and COVID-19.












