Cyclooxygenase (COX), officially known as prostaglandin-endoperoxide synthase (PTGS), is an enzyme that is responsible for biosynthesis of prostanoids, including thromboxane and prostaglandins such as prostacyclin, from arachidonic acid. A member of the animal-type heme peroxidase family, it is also known as prostaglandin G/H synthase. The specific reaction catalyzed is the conversion from arachidonic acid to prostaglandin H2 via a short-living prostaglandin G2 intermediate.

Eicosanoids are signaling molecules made by the enzymatic or non-enzymatic oxidation of arachidonic acid or other polyunsaturated fatty acids (PUFAs) that are, similar to arachidonic acid, around 20 carbon units in length. Eicosanoids are a sub-category of oxylipins, i.e. oxidized fatty acids of diverse carbon units in length, and are distinguished from other oxylipins by their overwhelming importance as cell signaling molecules. Eicosanoids function in diverse physiological systems and pathological processes such as: mounting or inhibiting inflammation, allergy, fever and other immune responses; regulating the abortion of pregnancy and normal childbirth; contributing to the perception of pain; regulating cell growth; controlling blood pressure; and modulating the regional flow of blood to tissues. In performing these roles, eicosanoids most often act as autocrine signaling agents to impact their cells of origin or as paracrine signaling agents to impact cells in the proximity of their cells of origin. Eicosanoids may also act as endocrine agents to control the function of distant cells.

Prostacyclin (also called prostaglandin I2 or PGI2) is a prostaglandin member of the eicosanoid family of lipid molecules. It inhibits platelet activation and is also an effective vasodilator.

Oxidative stress reflects an imbalance between the systemic manifestation of reactive oxygen species and a biological system's ability to readily detoxify the reactive intermediates or to repair the resulting damage. Disturbances in the normal redox state of cells can cause toxic effects through the production of peroxides and free radicals that damage all components of the cell, including proteins, lipids, and DNA. Oxidative stress from oxidative metabolism causes base damage, as well as strand breaks in DNA. Base damage is mostly indirect and caused by the reactive oxygen species generated, e.g., O2− (superoxide radical), OH (hydroxyl radical) and H2O2 (hydrogen peroxide). Further, some reactive oxidative species act as cellular messengers in redox signaling. Thus, oxidative stress can cause disruptions in normal mechanisms of cellular signaling.

Malondialdehyde belong to the class of β-dicarbonyls. A colorless liquid, malondialdehyde is a highly reactive compound that occurs as the enol. It is a physiological metabolite, and a marker for oxidative stress.

Prostaglandin E2 (PGE2), also known as dinoprostone, is a naturally occurring prostaglandin with oxytocic properties that is used as a medication. Dinoprostone is used in labor induction, bleeding after delivery, termination of pregnancy, and in newborn babies to keep the ductus arteriosus open. In babies it is used in those with congenital heart defects until surgery can be carried out. It is also used to manage gestational trophoblastic disease. It may be used within the vagina or by injection into a vein.
Most of the eicosanoid receptors are integral membrane protein G protein-coupled receptors (GPCRs) that bind and respond to eicosanoid signaling molecules. Eicosanoids are rapidly metabolized to inactive products and therefore are short-lived. Accordingly, the eicosanoid-receptor interaction is typically limited to a local interaction: cells, upon stimulation, metabolize arachidonic acid to an eicosanoid which then binds cognate receptors on either its parent cell or on nearby cells to trigger functional responses within a restricted tissue area, e.g. an inflammatory response to an invading pathogen. In some cases, however, the synthesized eicosanoid travels through the blood to trigger systemic or coordinated tissue responses, e.g. prostaglandin (PG) E2 released locally travels to the hypothalamus to trigger a febrile reaction. An example of a non-GPCR receptor that binds many eicosanoids is the PPAR-γ nuclear receptor.

Prostaglandin-I synthase also known as prostaglandin I2 (prostacyclin) synthase (PTGIS) or CYP8A1 is an enzyme involved in prostanoid biosynthesis that in humans is encoded by the PTGIS gene. This enzyme belongs to the family of cytochrome P450 isomerases.
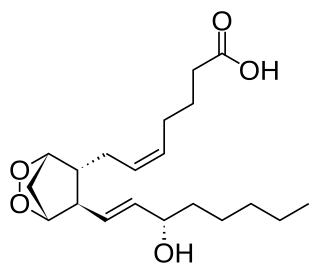
Prostaglandin H2 (PGH2), or prostaglandin H2 (PGH2), is a type of prostaglandin and a precursor for many other biologically significant molecules. It is synthesized from arachidonic acid in a reaction catalyzed by a cyclooxygenase enzyme. The conversion from arachidonic acid to prostaglandin H2 is a two-step process. First, COX-1 catalyzes the addition of two free oxygens to form the 1,2-dioxane bridge and a peroxide functional group to form prostaglandin G2 (PGG2). Second, COX-2 reduces the peroxide functional group to a secondary alcohol, forming prostaglandin H2. Other peroxidases like hydroquinone have been observed to reduce PGG2 to PGH2. PGH2 is unstable at room temperature, with a half life of 90-100 seconds, so it is often converted into a different prostaglandin.
Cyclopentenone prostaglandins are a subset of prostaglandins (PGs) or prostanoids that has 15-deoxy-Δ12,14-prostaglandin J2 (15-d-Δ12,14-PGJ2), Δ12-PGJ2, and PGJ2 as its most prominent members but also including PGA2, PGA1, and, while not classified as such, other PGs. 15-d-Δ12,14-PGJ2, Δ12-PGJ2, and PGJ2 share a common mono-unsaturated cyclopentenone structure as well as a set of similar biological activities including the ability to suppress inflammation responses and the growth as well as survival of cells, particularly those of cancerous or neurological origin. Consequently, these three cyclopentenone-PGs and the two epoxyisoprostanes are suggested to be models for the development of novel anti-inflammatory and anti-cancer drugs. The cyclopenentone prostaglandins are structurally and functionally related to a subset of isoprostanes viz., two cyclopentenone isoprostanes, 5,6-epoxyisoprostane E2 and 5,6-epoxisoprostane A2.

Cyclooxygenase-2 (COX-2), also known as Prostaglandin-endoperoxide synthase 2 (HUGO PTGS2), is an enzyme that in humans is encoded by the PTGS2 gene. In humans it is one of two cyclooxygenases. It is involved in the conversion of arachidonic acid to prostaglandin H2, an important precursor of prostacyclin, which is expressed in inflammation.

Cyclooxygenase 1 (COX-1), also known as prostaglandin-endoperoxide synthase 1, is an enzyme that in humans is encoded by the PTGS1 gene. In humans it is one of two cyclooxygenases.

Prostaglandin E2 receptor 1 (EP1) is a 42kDa prostaglandin receptor encoded by the PTGER1 gene. EP1 is one of four identified EP receptors, EP1, EP2, EP3, and EP4 which bind with and mediate cellular responses principally to prostaglandin E2) (PGE2) and also but generally with lesser affinity and responsiveness to certain other prostanoids (see Prostaglandin receptors). Animal model studies have implicated EP1 in various physiological and pathological responses. However, key differences in the distribution of EP1 between these test animals and humans as well as other complicating issues make it difficult to establish the function(s) of this receptor in human health and disease.

Prostaglandin E2 receptor 2, also known as EP2, is a prostaglandin receptor for prostaglandin E2 (PGE2) encoded by the human gene PTGER2: it is one of four identified EP receptors, the others being EP1, EP3, and EP4, which bind with and mediate cellular responses to PGE2 and also, but with lesser affinity and responsiveness, certain other prostanoids (see Prostaglandin receptors). EP has been implicated in various physiological and pathological responses.
Animal heme-dependent peroxidases is a family of peroxidases. Peroxidases are found in bacteria, fungi, plants and animals. On the basis of sequence similarity, a number of animal heme peroxidases can be categorized as members of a superfamily: myeloperoxidase (MPO); eosinophil peroxidase (EPO); lactoperoxidase (LPO); thyroid peroxidase (TPO); prostaglandin H synthase (PGHS); and peroxidasin.

Cytochrome P450 4F8 is a protein that in humans is encoded by the CYP4F8 gene.

Microsomal prostaglandin E synthase-1 (mPGES-1) or Prostaglandin E synthase is an enzyme that in humans is encoded by the PTGES gene.

12-Hydroxyheptadecatrienoic acid (also termed 12-HHT, 12(S)-hydroxyheptadeca-5Z,8E,10E-trienoic acid, or 12(S)-HHTrE) is a 17 carbon metabolite of the 20 carbon polyunsaturated fatty acid, arachidonic acid. It was discovered and structurally defined in 1973 by P. Wlodawer, Bengt I. Samuelsson, and M. Hamberg, as a product of arachidonic acid metabolism made by microsomes (i.e. endoplasmic reticulum) isolated from sheep seminal vesicle glands and by intact human platelets. 12-HHT is less ambiguously termed 12-(S)-hydroxy-5Z,8E,10E-heptadecatrienoic acid to indicate the S stereoisomerism of its 12-hydroxyl residue and the Z, E, and E cis-trans isomerism of its three double bonds. The metabolite was for many years thought to be merely a biologically inactive byproduct of prostaglandin synthesis. More recent studies, however, have attached potentially important activity to it.

The hydroxylation of estradiol is one of the major routes of metabolism of the estrogen steroid hormone estradiol. It is hydroxylated into the catechol estrogens 2-hydroxyestradiol and 4-hydroxyestradiol and into estriol (16α-hydroxyestradiol), reactions which are catalyzed by cytochrome P450 enzymes predominantly in the liver, but also in various other tissues.
In enzymology, a prostaglandin-F synthase (PGFS; EC 1.1.1.188) is an enzyme that catalyzes the chemical reaction:

















