Related Research Articles

Dehydroepiandrosterone (DHEA), also known as androstenolone, is an endogenous steroid hormone precursor. It is one of the most abundant circulating steroids in humans. DHEA is produced in the adrenal glands, the gonads, and the brain. It functions as a metabolic intermediate in the biosynthesis of the androgen and estrogen sex steroids both in the gonads and in various other tissues. However, DHEA also has a variety of potential biological effects in its own right, binding to an array of nuclear and cell surface receptors, and acting as a neurosteroid and modulator of neurotrophic factor receptors.

Progestogens, also sometimes written progestagens or gestagens, are a class of natural or synthetic steroid hormones that bind to and activate the progesterone receptors (PR). Progesterone is the major and most important progestogen in the body. The progestogens are named for their function in maintaining pregnancy, although they are also present at other phases of the estrous and menstrual cycles.

The adrenal cortex is the outer region and also the largest part of the adrenal gland. It is divided into three separate zones: zona glomerulosa, zona fasciculata and zona reticularis. Each zone is responsible for producing specific hormones. It is also a secondary site of androgen synthesis.

Transcortin, also known as corticosteroid-binding globulin (CBG) or serpin A6, is a protein produced in the liver in animals. In humans it is encoded by the SERPINA6 gene. It is an alpha-globulin.
Congenital adrenal hyperplasia due to 17α-hydroxylase deficiency is an uncommon form of congenital adrenal hyperplasia (CAH) resulting from a mutation in the gene CYP17A1, which produces the enzyme 17α-hydroxylase. It causes decreased synthesis of cortisol and sex hormones, with resulting increase in mineralocorticoid production. Thus, common symptoms include mild cortisol deficiency, ambiguous genitalia in men or amenorrhea at puberty in women, and hypokalemic hypertension. However, partial (incomplete) deficiency often has inconsistent symptoms between patients, and affected women may be asymptomatic except for infertility.
Pregnenolone (P5), or pregn-5-en-3β-ol-20-one, is an endogenous steroid and precursor/metabolic intermediate in the biosynthesis of most of the steroid hormones, including the progestogens, androgens, estrogens, glucocorticoids, and mineralocorticoids. In addition, pregnenolone is biologically active in its own right, acting as a neurosteroid.
11β-Hydroxysteroid dehydrogenase enzymes catalyze the conversion of inert 11 keto-products (cortisone) to active cortisol, or vice versa, thus regulating the access of glucocorticoids to the steroid receptors.

Dehydroepiandrosterone sulfate, abbreviated as DHEA sulfate or DHEA-S, also known as androstenolone sulfate, is an endogenous androstane steroid that is produced by the adrenal cortex. It is the 3β-sulfate ester and a metabolite of dehydroepiandrosterone (DHEA) and circulates in far greater relative concentrations than DHEA. The steroid is hormonally inert and is instead an important neurosteroid and neurotrophin.
3β-Hydroxysteroid dehydrogenase/Δ5-4 isomerase (3β-HSD) is an enzyme that catalyzes the biosynthesis of the steroid progesterone from pregnenolone, 17α-hydroxyprogesterone from 17α-hydroxypregnenolone, and androstenedione from dehydroepiandrosterone (DHEA) in the adrenal gland. It is the only enzyme in the adrenal pathway of corticosteroid synthesis that is not a member of the cytochrome P450 family. It is also present in other steroid-producing tissues, including the ovary, testis and placenta. In humans, there are two 3β-HSD isozymes encoded by the HSD3B1 and HSD3B2 genes.

Steroid 11β-hydroxylase, also known as steroid 11β-monooxygenase, is a steroid hydroxylase found in the zona glomerulosa and zona fasciculata of the adrenal cortex. Named officially the cytochrome P450 11B1, mitochondrial, it is a protein that in humans is encoded by the CYP11B1 gene. The enzyme is involved in the biosynthesis of adrenal corticosteroids by catalyzing the addition of hydroxyl groups during oxidation reactions.
11-Deoxycortisol, also known as cortodoxone (INN), cortexolone as well as 17α,21-dihydroxyprogesterone or 17α,21-dihydroxypregn-4-ene-3,20-dione, is an endogenous glucocorticoid steroid hormone, and a metabolic intermediate toward cortisol. It was first described by Tadeusz Reichstein in 1938 as Substance S, thus has also been referred to as Reichstein's Substance S or Compound S.
Critical illness–related corticosteroid insufficiency is a form of adrenal insufficiency in critically ill patients who have blood corticosteroid levels which are inadequate for the severe stress response they experience. Combined with decreased glucocorticoid receptor sensitivity and tissue response to corticosteroids, this adrenal insufficiency constitutes a negative prognostic factor for intensive care patients.
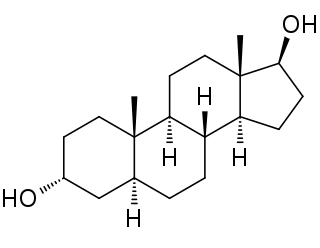
3α-Androstanediol also known as 5α-androstane-3α,17β-diol and sometimes shortened in the literature to 3α-diol, is an endogenous steroid hormone and neurosteroid and a metabolite of androgens like dihydrotestosterone (DHT).

Roxibolone (INN), also known as 11β,17β-dihydroxy-17α-methyl-3-oxoandrosta-1,4-diene-2-carboxylic acid, is a steroidal antiglucocorticoid described as an anticholesterolemic (cholesterol-lowering) and anabolic drug which was never marketed. Roxibolone is closely related to formebolone, which shows antiglucocorticoid activity similarly and, with the exception of having a carboxaldehyde group at the C2 position instead of a carboxylic acid group, roxibolone is structurally almost identical to. The 2-decyl ester of roxibolone, decylroxibolone, is a long-acting prodrug of roxibolone with similar activity.

21-Deoxycortisone, also known as 21-desoxycortisone, 11-keto-17α-hydroxyprogesterone, or 17α-hydroxypregn-4-ene-3,11,20-trione, is a naturally occurring, endogenous steroid and minor intermediate and metabolite in corticosteroid metabolism. It is related to 21-deoxycortisol (11β,17α-dihydroxyprogesterone) and is reversibly formed from it by 11β-hydroxysteroid dehydrogenase, analogously to the reversible formation of cortisone from cortisol. 21-Deoxycortisone can be transformed into cortisone by 21-hydroxylase.
Adrenal steroids are steroids that are derived from the adrenal glands. They include corticosteroids, which consist of glucocorticoids like cortisol and mineralocorticoids like aldosterone, adrenal androgens like dehydroepiandrosterone (DHEA), DHEA sulfate (DHEA-S), and androstenedione (A4), and neurosteroids like DHEA and DHEA-S, as well as pregnenolone and pregnenolone sulfate (P5-S). Adrenal steroids are specifically produced in the adrenal cortex.

The pharmacodynamics of spironolactone, an antimineralocorticoid and antiandrogen medication, concern its mechanisms of action, including its biological targets and activities, as well as its physiological effects. The pharmacodynamics of spironolactone are characterized by high antimineralocorticoid activity, moderate antiandrogenic activity, and weak steroidogenesis inhibition. In addition, spironolactone has sometimes been found to increase estradiol and cortisol levels and hence could have slight indirect estrogenic and glucocorticoid effects. The medication has also been found to interact very weakly with the estrogen and progesterone receptors, and to act as an agonist of the pregnane X receptor. Likely due to increased activation of the estrogen and/or progesterone receptors, spironolactone has very weak but significant antigonadotropic effects.
Late onset congenital adrenal hyperplasia (LOCAH), also known as nonclassic congenital adrenal hyperplasia, is a milder form of congenital adrenal hyperplasia (CAH), a group of autosomal recessive disorders characterized by impaired cortisol synthesis that leads to variable degrees of postnatal androgen excess.

The androgen backdoor pathway synthesizes physiologically relevant androgens from 21-carbon steroids (pregnanes) via 5α-reduction, bypassing testosterone. This differs from the conventional, canonical androgenic pathway, which involves testosterone.
Adrenal androgen stimulating hormone (AASH), also known as cortical androgen stimulating hormone (CASH), is a hypothetical hormone which has been proposed to stimulate the adrenal glands to produce adrenal androgens such as dehydroepiandrosterone (DHEA), dehydroepiandrosterone sulfate (DHEA-S), and androstenedione (A4). It is hypothesized to be involved in adrenarche and adrenopause. The existence of this hormone is controversial and disputed and it has not been identified to date. A number of other mechanisms and/or hormones may instead play the functional role of the so-called AASH.
References
- ↑ Needham D, McIntosh TJ, Evans E (1988). "Thermomechanical and transition properties of dimyristoylphosphatidylcholine/cholesterol bilayers". Biochemistry. 27 (13): 4668–73. doi:10.1021/bi00413a013. PMID 3167010.
- ↑ Henson MC, Pepe GJ, Albrecht ED (1987). "Transuterofetoplacental conversion of pregnenolone to progesterone in antiestrogen-treated baboons". Endocrinology. 121 (4): 1265–71. doi:10.1210/endo-121-4-1265. PMID 3653027.
- 1 2 3 Spinola PG, Marchetti B, Mérand Y, Bélanger A, Labrie F (1988). "Effects of the aromatase inhibitor 4-hydroxyandrostenedione and the antiandrogen flutamide on growth and steroid levels in DMBA-induced rat mammary tumors". Breast Cancer Res. Treat. 12 (3): 287–96. doi:10.1007/BF01811241. PMID 3147727. S2CID 2197068.
- 1 2 3 4 Martikainen H, Rönnberg L, Ruokonen A, Vihko R (1985). "Testicular responsiveness to hCG before and after long-term antiestrogen treatment in oligozoospermic men". J. Steroid Biochem. 23 (5A): 651–5. doi:10.1016/0022-4731(85)90017-2. PMID 4079381.
- 1 2 Miura T, Higuchi M, Ozaki Y, Ohta T, Miura C (2006). "Progestin is an essential factor for the initiation of the meiosis in spermatogenetic cells of the eel". Proc. Natl. Acad. Sci. U.S.A. 103 (19): 7333–8. Bibcode:2006PNAS..103.7333M. doi: 10.1073/pnas.0508419103 . PMC 1464341 . PMID 16651536.
- 1 2 3 Raimondi SG, Olivier NS, Patrito LC, Flury A (1989). "Regulation of the 3 beta-hydroxysteroid dehydrogenase activity in tissue fragments and microsomes from human term placenta: kinetic analysis and inhibition by steroids". J. Steroid Biochem. 32 (3): 413–20. doi:10.1016/0022-4731(89)90215-X. PMID 2523011.
- 1 2 3 4 5 6 Sippell WG, Dörr HG, Bidlingmaier F, Knorr D (1980). "Plasma levels of aldosterone, corticosterone, 11-deoxycorticosterone, progesterone, 17-hydroxyprogesterone, cortisol, and cortisone during infancy and childhood". Pediatr. Res. 14 (1): 39–46. doi: 10.1203/00006450-198014010-00010 . PMID 7360520.
- 1 2 Bridges NA, Hindmarsh PC, Pringle PJ, Honour JW, Brook CG (1998). "Cortisol, androstenedione (A4), dehydroepiandrosterone sulphate (DHEAS) and 17 hydroxyprogesterone (17OHP) responses to low doses of (1-24)ACTH". J. Clin. Endocrinol. Metab. 83 (10): 3750–3. doi: 10.1210/jcem.83.10.5315 . PMID 9768696.
- 1 2 Harding CF, Sheridan K, Walters MJ (1983). "Hormonal specificity and activation of sexual behavior in male zebra finches". Hormones and Behavior. 17 (1): 111–33. doi:10.1016/0018-506X(83)90021-1. PMID 6862388. S2CID 29041025.
- ↑ Desprez D.1, Geraz E., Hoareau M.C., Melard C., Bosc P., Baroiller J.F. (2003). "Production of a high percentage of male offspring with a natural androgen, 11beta-hydroxyandrostenedione (11betaOHA4), in Florida red tilapia" (PDF). Aquaculture. 216 (1): 55–65. Bibcode:2003Aquac.216...55D. doi:10.1016/S0044-8486(02)00276-4. hdl:2268/76893.
{{cite journal}}: CS1 maint: multiple names: authors list (link) CS1 maint: numeric names: authors list (link) - ↑ Govoroun M, McMeel OM, D'Cotta H, et al. (2001). "Steroid enzyme gene expressions during natural and androgen-induced gonadal differentiation in the rainbow trout, Oncorhynchus mykiss". J. Exp. Zool. 290 (6): 558–66. Bibcode:2001JEZ...290..558G. doi:10.1002/jez.1106. PMID 11748604.
- 1 2 D. Consten, E.D. Keuning, M. Terlou, J.G.D. Lambert, H.J.Th. Goos (2001). "Cortisol effects on the testicular androgen synthesizing capacity in common carp, Cyprinus carpio L.". Fish Physiology and Biochemistry. 25 (2): 91–98. doi:10.1023/A:1020538729104. S2CID 25897842.
- ↑ Matthiesson KL, Stanton PG, O'Donnell L, et al. (2005). "Effects of testosterone and levonorgestrel combined with a 5alpha-reductase inhibitor or gonadotropin-releasing hormone antagonist on spermatogenesis and intratesticular steroid levels in normal men". J. Clin. Endocrinol. Metab. 90 (10): 5647–55. doi: 10.1210/jc.2005-0639 . PMID 16030154.
- ↑ Pringle AK, Schmidt W, Deans JK, Wulfert E, Reymann KG, Sundstrom LE (2003). "7-Hydroxylated epiandrosterone (7-OH-EPIA) reduces ischaemia-induced neuronal damage both in vivo and in vitro". Eur. J. Neurosci. 18 (1): 117–24. doi:10.1046/j.1460-9568.2003.02734.x. PMID 12859344. S2CID 42753764.
- 1 2 Labrie C, Simard J, Zhao HF, Belanger A, Pelletier G, Labrie F (1989). "Stimulation of androgen-dependent gene expression by the adrenal precursors dehydroepiandrosterone and androstenedione in the rat ventral prostate". Endocrinology. 124 (6): 2745–54. doi:10.1210/endo-124-6-2745. PMID 2524377.
- 1 2 Dumitrescu A, Aberdeen GW, Pepe GJ, Albrecht ED (2007). "Developmental expression of cell cycle regulators in the baboon fetal adrenal gland". J. Endocrinol. 192 (1): 237–47. doi: 10.1677/joe.1.06769 . PMID 17210761.
- ↑ Nuck BA, Lucky AW (1987). "Epitestosterone: a potential new antiandrogen". J. Invest. Dermatol. 89 (2): 209–11. doi: 10.1111/1523-1747.ep12470564 . PMID 3598212.
- 1 2 Perusquía M (2003). "Androgen-induced vasorelaxation: a potential vascular protective effect". Exp. Clin. Endocrinol. Diabetes. 111 (2): 55–9. doi:10.1055/s-2003-39229. PMID 12746753.
- ↑ T.T. Wong, Y. Zohar (2003). "The involvement of gonadotropin receptors in sex reversal: expression, distribution and regulation of gonadal FSH and LH receptors in the gilthead seabream". Fish Physiology and Biochemistry. 28 (1–4): 179–180. Bibcode:2003FPBio..28..179W. doi:10.1023/B:FISH.0000030520.60240.cd. S2CID 11881590.
- ↑ Lokman PM, Harris B, Kusakabe M, et al. (2002). "11-Oxygenated androgens in female teleosts: prevalence, abundance, and life history implications". Gen. Comp. Endocrinol. 129 (1): 1–12. doi:10.1016/S0016-6480(02)00562-2. PMID 12409090.
- ↑ Thomas RM, Urban JH, Peterson DA (2006). "Acute exposure to predator odor elicits a robust increase in corticosterone and a decrease in activity without altering proliferation in the adult rat hippocampus". Exp. Neurol. 201 (2): 308–15. doi:10.1016/j.expneurol.2006.04.010. PMID 16750196. S2CID 43129386.
- ↑ Reardon GE, Caldarella AM, Canalis E (1979). "Determination of serum cortisol and 11-deoxycortisol by liquid chromatography" (PDF). Clin. Chem. 25 (1): 122–6. doi:10.1093/clinchem/25.1.122. PMID 761348.
- ↑ Auchus RJ, Chandler DW, Singeetham S, et al. (2007). "Measurement of 18-Hydroxycorticosterone During Adrenal Vein Sampling for Primary Aldosteronism". Journal of Clinical Endocrinology & Metabolism. 92 (7): 2648–51. doi: 10.1210/jc.2006-2631 . PMID 17473070.
- ↑ Krishnan AV, Zhao XY, Swami S, et al. (2002). "A glucocorticoid-responsive mutant androgen receptor exhibits unique ligand specificity: therapeutic implications for androgen-independent prostate cancer". Endocrinology. 143 (5): 1889–1900. doi: 10.1210/endo.143.5.8778 . PMID 11956172.
- ↑ Nunez S, Trant JM (1999). "Regulation of interrenal gland steroidogenesis in the Atlantic stingray (Dasyatis sabina)". J. Exp. Zool. 284 (5): 517–25. doi:10.1002/(SICI)1097-010X(19991001)284:5<517::AID-JEZ7>3.0.CO;2-S. PMID 10469989.
- ↑ Kornel L, Smoszna-Konaszewska B (1995). "Aldosterone (ALDO) increases transmembrane influx of Na+ in vascular smooth muscle (VSM) cells through increased synthesis of Na+ channels". Steroids. 60 (1): 114–9. doi:10.1016/0039-128X(94)00016-6. PMID 7792795. S2CID 25467757.