Hypogonadism means diminished functional activity of the gonads—the testes or the ovaries—that may result in diminished production of sex hormones. Low androgen levels are referred to as hypoandrogenism and low estrogen as hypoestrogenism. These are responsible for the observed signs and symptoms in both males and females.
Kallmann syndrome (KS) is a genetic disorder that prevents a person from starting or fully completing puberty. Kallmann syndrome is a form of a group of conditions termed hypogonadotropic hypogonadism. To distinguish it from other forms of hypogonadotropic hypogonadism, Kallmann syndrome has the additional symptom of a total lack of sense of smell (anosmia) or a reduced sense of smell. If left untreated, people will have poorly defined secondary sexual characteristics, show signs of hypogonadism, almost invariably are infertile and are at increased risk of developing osteoporosis. A range of other physical symptoms affecting the face, hands and skeletal system can also occur.

Trestolone, also known as 7α-methyl-19-nortestosterone (MENT), is an experimental androgen/anabolic steroid (AAS) and progestogen medication which has been under development for potential use as a form of hormonal birth control for men and in androgen replacement therapy for low testosterone levels in men but has never been marketed for medical use. It is given as an implant that is placed into fat. As trestolone acetate, an androgen ester and prodrug of trestolone, the medication can also be given by injection into muscle.
Gepirone is an antidepressant and anxiolytic drug of the azapirone group that was synthesized by Bristol-Myers Squibb in 1986 and has been under development for the treatment of depression but has yet to be marketed. It has been under development in the U.S. in an extended release form, but despite completing phase III clinical trials and demonstrating efficacy, it has been rejected multiple times by the Food and Drug Administration (FDA) during the drug approval process. However, in March 2016, the FDA reversed course and ruled favorably on the efficacy of gepirone.
Enclomifene (INN), or enclomiphene (USAN), a nonsteroidal selective estrogen receptor modulator of the triphenylethylene group acts by antagonizing the estrogen receptor (ER) in the pituitary gland, which reduces negative feedback by estrogen on the hypothalamic-pituitary-gonadal axis, thereby increasing gonadotropin secretion and hence gonadal production of testosterone. It is one of the two stereoisomers of clomifene, which itself is a mixture of 38% zuclomifene and 62% enclomifene. Enclomifene is the (E)-stereoisomer of clomifene, while zuclomifene is the (Z)-stereoisomer. Whereas zuclomifene is more estrogenic, enclomifene is more antiestrogenic. In accordance, unlike enclomifene, zuclomifene is antigonadotropic due to activation of the ER and reduces testosterone levels in men. As such, isomerically pure enclomifene is more favorable than clomifene as a progonadotropin for the treatment of male hypogonadism.

The KiSS1-derived peptide receptor is a G protein-coupled receptor which binds the peptide hormone kisspeptin (metastin). Kisspeptin is encoded by the metastasis suppressor gene KISS1, which is expressed in a variety of endocrine and gonadal tissues. Activation of the kisspeptin receptor is linked to the phospholipase C and inositol trisphosphate second messenger cascades inside the cell.
PL-6983 is a synthetic peptide and selective MC4 receptor agonist which is under development by Palatin Technologies for the treatment of female sexual dysfunction and erectile dysfunction. It was developed as a successor to/replacement of bremelanotide (PT-141) due to concerns of the side effect of increased blood pressure seen with the latter in clinical trials. Relative to bremelanotide, PL-6983 produces significantly lower increases in blood pressure in animal models. The drug has reportedly been in pre-clinical development for all medical indications since 2008. Palatin has stated that "We are focusing development efforts on bremelanotide for [female sexual dysfunction], but are continuing evaluation of PL-6983." The chemical structure of PL-6983 has yet to be made public.
Hypogonadotropic hypogonadism (HH), is due to problems with either the hypothalamus or pituitary gland affecting the hypothalamic-pituitary-gonadal axis. Hypothalamic disorders result from a deficiency in the release of gonadotropic releasing hormone (GnRH), while pituitary gland disorders are due to a deficiency in the release of gonadotropins from the anterior pituitary. GnRH is the central regulator in reproductive function and sexual development via the HPG axis. GnRH is released by GnRH neurons, which are hypothalamic neuroendocrine cells, into the hypophyseal portal system acting on gonadotrophs in the anterior pituitary. The release of gonadotropins, LH and FSH, act on the gonads for the development and maintenance of proper adult reproductive physiology. LH acts on Leydig cells in the male testes and theca cells in the female. FSH acts on Sertoli cells in the male and follicular cells in the female. Combined this causes the secretion of gonadal sex steroids and the initiation of folliculogenesis and spermatogenesis. The production of sex steroids forms a negative feedback loop acting on both the anterior pituitary and hypothalamus causing a pulsatile secretion of GnRH. GnRH neurons lack sex steroid receptors and mediators such as kisspeptin stimulate GnRH neurons for pulsatile secretion of GnRH.

Samidorphan, is an opioid antagonist which in the form of olanzapine/samidorphan is used in the treatment of schizophrenia and bipolar disorder. Samidorphan reduces the weight gain associated with olanzapine. Samidorphan is taken by mouth.

PF-592,379 is a drug developed by Pfizer which acts as a potent, selective and orally active agonist for the dopamine D3 receptor, which is under development as a potential medication for the treatment of female sexual dysfunction and male erectile dysfunction. Unlike some other less selective D3 agonists, a research study showed that PF-592,379 has little abuse potential in animal studies, and so was selected for further development and potentially human clinical trials. Development has since been discontinued.
TGBA01AD (also known as FKB01MD) is a serotonin reuptake inhibitor, 5-HT1A and 5-HT1D receptor agonist, and 5-HT2 receptor antagonist which is under development by Fabre-Kramer for the treatment of major depressive disorder. It has been in phase II clinical trials since 2009, and as of January 2016, remains in this phase of development.

Erteberel is a synthetic, nonsteroidal estrogen which acts as a selective ERβ agonist and is under development by Eli Lilly for the treatment of schizophrenia. It is specifically under investigation for the treatment of negative symptoms and cognitive impairment associated with the condition. As of 2015, it is in phase II clinical trials for this indication in the United States. Erteberel was also under investigation for the treatment of benign prostatic hyperplasia and reached phase II clinical studies for this use but failed to improve symptoms in men with the condition and development for this indication was discontinued. The drug has also been proposed as a potential novel treatment for glioblastoma.

Fispemifene is a nonsteroidal selective estrogen receptor modulator (SERM) of the triphenylethylene group that was developed for the treatment of male hypogonadism but was abandoned and never marketed. It reached phase II clinical trials for this indication before development was terminated in March 2016. The drug failed to achieve statistical significance on key effectiveness endpoints in clinical trials and was discontinued by its developer for strategic reasons.
Androstanolone, or stanolone, also known as dihydrotestosterone (DHT) and sold under the brand name Andractim among others, is an androgen and anabolic steroid (AAS) medication and hormone which is used mainly in the treatment of low testosterone levels in men. It is also used to treat breast development and small penis in males. It is typically given as a gel for application to the skin, but can also be used as an ester by injection into muscle.
Dagrocorat is a nonsteroidal but steroid-like selective glucocorticoid receptor modulator (SGRM) which was under development for the treatment of rheumatoid arthritis but was never marketed. It is described as a partial agonist and "dissociable" agonist of the glucocorticoid receptor. The drug reached phase I clinical trials prior to the discontinuation of its development. The C2α dihydrogen phosphate ester of dagrocorat, fosdagrocorat, was also under investigation, but its development was terminated as well.
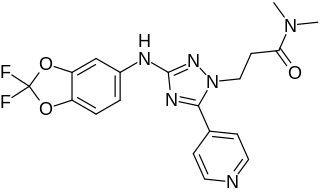
JNJ-39393406 is an experimental medication which is under development by Janssen Pharmaceutica, a division of Johnson & Johnson, for the treatment of depressive disorders and smoking withdrawal. It acts as a selective positive allosteric modulator of the α7 nicotinic acetylcholine receptor (nAChR). It does not act on the α4β2 or α3β4 nAChRs or the serotonin 5-HT3 receptor, and does not interact with a panel of 62 other receptors and enzymes. The drug has been found to lower the agonist and nicotine threshold for activation of the α7 nAChR by 10- to 20-fold and to increase the maximum agonist response of the α7 nAChR by 17- to 20-fold.

Hypidone (developmental code name YL-0919) is an investigational serotonergic antidepressant which is under development for the treatment of major depressive disorder. It acts as a serotonin reuptake inhibitor, 5-HT1A receptor partial agonist, and 5-HT6 receptor full agonist. It is used as the hydrochloride salt. As of January 2021, hypidone is in phase 2 clinical trials for major depressive disorder.












