
Hepatitis is inflammation of the liver tissue. Some people or animals with hepatitis have no symptoms, whereas others develop yellow discoloration of the skin and whites of the eyes (jaundice), poor appetite, vomiting, tiredness, abdominal pain, and diarrhea. Hepatitis is acute if it resolves within six months, and chronic if it lasts longer than six months. Acute hepatitis can resolve on its own, progress to chronic hepatitis, or (rarely) result in acute liver failure. Chronic hepatitis may progress to scarring of the liver (cirrhosis), liver failure, and liver cancer.

Hepatitis C is an infectious disease caused by the hepatitis C virus (HCV) that primarily affects the liver; it is a type of viral hepatitis. During the initial infection period, people often have mild or no symptoms. Early symptoms can include fever, dark urine, abdominal pain, and yellow tinged skin. The virus persists in the liver, becoming chronic, in about 70% of those initially infected. Early on, chronic infection typically has no symptoms. Over many years however, it often leads to liver disease and occasionally cirrhosis. In some cases, those with cirrhosis will develop serious complications such as liver failure, liver cancer, or dilated blood vessels in the esophagus and stomach.
Liver function tests, also referred to as a hepatic panel, are groups of blood tests that provide information about the state of a patient's liver. These tests include prothrombin time (PT/INR), activated partial thromboplastin time (aPTT), albumin, bilirubin, and others. The liver transaminases aspartate transaminase and alanine transaminase are useful biomarkers of liver injury in a patient with some degree of intact liver function.

Hepatocellular carcinoma (HCC) is the most common type of primary liver cancer in adults and is currently the most common cause of death in people with cirrhosis. HCC is the third leading cause of cancer-related deaths worldwide.

Alcoholic liver disease (ALD), also called alcohol-related liver disease (ARLD), is a term that encompasses the liver manifestations of alcohol overconsumption, including fatty liver, alcoholic hepatitis, and chronic hepatitis with liver fibrosis or cirrhosis.

Alcoholic hepatitis is hepatitis due to excessive intake of alcohol. Patients typically have a history of at least 10 years of heavy alcohol intake, typically 8–10 drinks per day. It is usually found in association with fatty liver, an early stage of alcoholic liver disease, and may contribute to the progression of fibrosis, leading to cirrhosis. Symptoms may present acutely after a large amount of alcoholic intake in a short time period, or after years of excess alcohol intake. Signs and symptoms of alcoholic hepatitis include jaundice, ascites, fatigue and hepatic encephalopathy. Mild cases are self-limiting, but severe cases have a high risk of death. Severity in alcoholic hepatitis is determined several clinical prediction models such as the Maddrey's Discriminant Function and the MELD score.

Autoimmune hepatitis, formerly known as lupoid hepatitis, plasma cell hepatitis, or autoimmune chronic active hepatitis, is a chronic, autoimmune disease of the liver that occurs when the body's immune system attacks liver cells, causing the liver to be inflamed. Common initial symptoms may include fatigue, nausea, muscle aches, or weight loss or signs of acute liver inflammation including fever, jaundice, and right upper quadrant abdominal pain. Individuals with autoimmune hepatitis often have no initial symptoms and the disease may be detected by abnormal liver function tests and increased protein levels during routine bloodwork or the observation of an abnormal-looking liver during abdominal surgery.

Primary biliary cholangitis (PBC), previously known as primary biliary cirrhosis, is an autoimmune disease of the liver. It results from a slow, progressive destruction of the small bile ducts of the liver, causing bile and other toxins to build up in the liver, a condition called cholestasis. Further slow damage to the liver tissue can lead to scarring, fibrosis, and eventually cirrhosis.

Liver disease, or hepatic disease, is any of many diseases of the liver. If long-lasting it is termed chronic liver disease. Although the diseases differ in detail, liver diseases often have features in common.

Fatty liver disease (FLD), also known as hepatic steatosis and steatotic liver disease (SLD), is a condition where excess fat builds up in the liver. Often there are no or few symptoms. Occasionally there may be tiredness or pain in the upper right side of the abdomen. Complications may include cirrhosis, liver cancer, and esophageal varices.

Steatohepatitis is a type of fatty liver disease, characterized by inflammation of the liver with concurrent fat accumulation in liver. Mere deposition of fat in the liver is termed steatosis, and together these constitute fatty liver changes.

Metabolic dysfunction–associated steatotic liver disease (MASLD), previously known as non-alcoholic fatty liver disease (NAFLD), is a type of chronic liver disease. This condition is diagnosed when there is excessive fat build-up in the liver, and at least one metabolic risk factor. When there is also increased alcohol intake, the term MetALD, or metabolic dysfunction and alcohol associated/related liver disease is used, and differentiated from alcohol-related liver disease (ALD) where alcohol is the predominant cause of the steatotic liver disease. The terms non-alcoholic fatty liver (NAFL) and non-alcoholic steatohepatitis have been used to describe different severities, the latter indicating the presence of further liver inflammation. NAFL is less dangerous than NASH and usually does not progress to it, but this progression may eventually lead to complications, such as cirrhosis, liver cancer, liver failure, and cardiovascular disease.

Liver failure is the inability of the liver to perform its normal synthetic and metabolic functions as part of normal physiology. Two forms are recognised, acute and chronic (cirrhosis). Recently, a third form of liver failure known as acute-on-chronic liver failure (ACLF) is increasingly being recognized.

Cirrhosis, also known as liver cirrhosis or hepatic cirrhosis, and end-stage liver disease, is a condition of the liver in which the normal functioning tissue, or parenchyma, is replaced with scar tissue (fibrosis) and regenerative nodules as a result of chronic liver disease. Damage to the liver leads to repair of liver tissue and subsequent formation of scar tissue. Over time, scar tissue and nodules of regenerating hepatocytes can replace the parenchyma, causing increased resistance to blood flow in the liver's capillaries—the hepatic sinusoids—and consequently portal hypertension, as well as impairment in other aspects of liver function. The disease typically develops slowly over months or years.
The European Association for the Study of the Liver (EASL), founded in 1966, is a medical association dedicated to pursuing excellence in liver research, to the clinical practice of liver disorders, and to providing education to all those interested in hepatology. As of 2024, EASL serves 7,000 members from 112 countries.
Thomas D. Schiano is an American specialist in liver transplantation, intestinal transplantation and in the diagnosis and treatment of acute and chronic liver disease. He serves as associate editor for the journals Hepatology and Liver Transplantation and has published more than 200 peer-reviewed articles and abstracts and more than 20 book chapters.
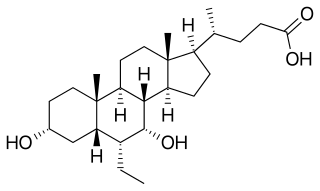
Obeticholic acid (OCA), sold under the brand name Ocaliva, is a semi-synthetic bile acid analogue which has the chemical structure 6α-ethyl-chenodeoxycholic acid. It is used as a medication used to treat primary biliary cholangitis. Intercept Pharmaceuticals Inc. hold the worldwide rights to develop OCA outside Japan and China, where it is licensed to Dainippon Sumitomo Pharma.
Detlef Schuppan is a German biochemist and physician. He focuses on the diagnosis and treatment of coeliac disease and wheat sensitivity, fibrotic liver diseases and the immunology of chronic diseases and cancer. He is the director of the Institute of Translational Immunology and a professor of internal medicine, gastroenterology, and hepatology at the Medical Center of the Johannes Gutenberg University of Mainz in Germany. He directs the outpatient clinic for coeliac disease and small intestinal diseases. He is also a professor of medicine and a senior visiting scientist at Harvard Medical School.
Hyperbilirubinemia is a clinical condition describing an elevation of blood bilirubin level due to the inability to properly metabolise or excrete bilirubin, a product of erythrocytes breakdown. In severe cases, it is manifested as jaundice, the yellowing of tissues like skin and the sclera when excess bilirubin deposits in them. The US records 52,500 jaundice patients annually. By definition, bilirubin concentration of greater than 3 mg/ml is considered hyperbilirubinemia, following which jaundice progressively develops and becomes apparent when plasma levels reach 20 mg/ml. Rather than a disease itself, hyperbilirubinemia is indicative of multifactorial underlying disorders that trace back to deviations from regular bilirubin metabolism. Diagnosis of hyperbilirubinemia depends on physical examination, urinalysis, serum tests, medical history and imaging to identify the cause. Genetic diseases, alcohol, pregnancy and hepatitis viruses affect the likelihood of hyperbilirubinemia. Causes of hyperbilirubinemia mainly arise from the liver. These include haemolytic anaemias, enzymatic disorders, liver damage and gallstones. Hyperbilirubinemia itself is often benign. Only in extreme cases does kernicterus, a type of brain injury, occur. Therapy for adult hyperbilirubinemia targets the underlying diseases but patients with jaundice often have poor outcomes.
Nimer Assy is an Israeli hepatologist and academic focusing on internal medicine and liver transplantation. He is a professor at the Bar-Ilan University Azrieli Medical School and the Department Head of the Clinical Research Unit within Internal Medicine Ward A of the Galilee Medical Center.













