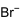| |||
 Methylammonium bromide crystals | |||
| Names | |||
|---|---|---|---|
| IUPAC name Methylazanium bromide | |||
| Systematic IUPAC name Methanaminium bromide | |||
Other names
| |||
| Identifiers | |||
3D model (JSmol) | |||
| ChemSpider | |||
| ECHA InfoCard | 100.027.255 | ||
| EC Number |
| ||
PubChem CID | |||
CompTox Dashboard (EPA) | |||
| |||
| |||
| Properties | |||
| CH3NH3Br | |||
| Molar mass | 111.96904 g/mol | ||
| Appearance | White crystals [1] | ||
| Melting point | 296 [2] °C (565 °F; 569 K) | ||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
Main hazards | irritant | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
Methylammonium bromide in an organic halide with the formula of CH3NH3Br. It is the salt of methylammonium and bromide. It is a colorless, water-soluble solid.
The methylammonium halides are precursors to perovskite solar cells, which are being evaluated. [3]