Related Research Articles

Parathyroid hormone(PTH),also called parathormone or parathyrin,is a peptide hormone secreted by the parathyroid glands that regulates the serum calcium concentration through its effects on bone,kidney,and intestine.
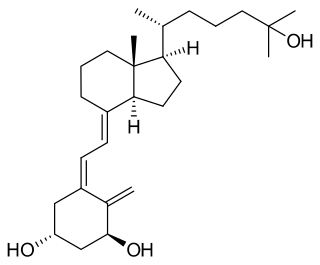
Osteomalacia is a disease characterized by the softening of the bones caused by impaired bone metabolism primarily due to inadequate levels of available phosphate,calcium,and vitamin D,or because of resorption of calcium. The impairment of bone metabolism causes inadequate bone mineralization.

Hyperparathyroidism is an increase in parathyroid hormone (PTH) levels in the blood. This occurs from a disorder either within the parathyroid glands or as response to external stimuli. Symptoms of hyperparathyroidism are caused by inappropriately normal or elevated blood calcium excreted from the bones and flowing into the blood stream in response to increased production of parathyroid hormone. In healthy people,when blood calcium levels are high,parathyroid hormone levels should be low. With long-standing hyperparathyroidism,the most common symptom is kidney stones. Other symptoms may include bone pain,weakness,depression,confusion,and increased urination. Both primary and secondary may result in osteoporosis.

Calcitriol is a hormone and the active form of vitamin D,normally made in the kidney. It is also known as 1,25-dihydroxycholecalciferol. It binds to and activates the vitamin D receptor in the nucleus of the cell,which then increases the expression of many genes. Calcitriol increases blood calcium mainly by increasing the uptake of calcium from the intestines.
Renal osteodystrophy is currently defined as an alteration of bone morphology in patients with chronic kidney disease (CKD). It is one measure of the skeletal component of the systemic disorder of chronic kidney disease-mineral and bone disorder (CKD-MBD). The term "renal osteodystrophy" was coined in 1943,60 years after an association was identified between bone disease and kidney failure.

Primary hyperparathyroidism is a medical condition where the parathyroid gland produce excess amounts of parathyroid hormone (PTH). The symptoms of the condition relate to the resulting elevated serum calcium (hypercalcemia),which can cause digestive symptoms,kidney stones,psychiatric abnormalities,and bone disease.

Hypophosphatasia (;also called deficiency of alkaline phosphatase,phosphoethanolaminuria,or Rathbun's syndrome;sometimes abbreviated HPP) is a rare,and sometimes fatal,inherited metabolic bone disease. Clinical symptoms are heterogeneous,ranging from the rapidly fatal,perinatal variant,with profound skeletal hypomineralization,respiratory compromise or vitamin B6 dependent seizures to a milder,progressive osteomalacia later in life. Tissue non-specific alkaline phosphatase (TNSALP) deficiency in osteoblasts and chondrocytes impairs bone mineralization,leading to rickets or osteomalacia. The pathognomonic finding is subnormal serum activity of the TNSALP enzyme,which is caused by one of 388 genetic mutations identified to date,in the gene encoding TNSALP. Genetic inheritance is autosomal recessive for the perinatal and infantile forms but either autosomal recessive or autosomal dominant in the milder forms.
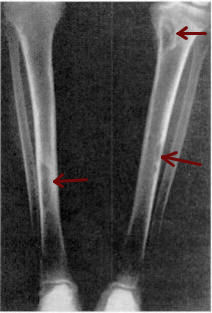
Osteitis fibrosa cystica is a skeletal disorder resulting in a loss of bone mass,a weakening of the bones as their calcified supporting structures are replaced with fibrous tissue,and the formation of cyst-like brown tumors in and around the bone. Osteitis fibrosis cystica (OFC),also known as osteitis fibrosa,osteodystrophia fibrosa,and von Recklinghausen's disease of bone,is caused by hyperparathyroidism,which is a surplus of parathyroid hormone from over-active parathyroid glands. This surplus stimulates the activity of osteoclasts,cells that break down bone,in a process known as osteoclastic bone resorption. The hyperparathyroidism can be triggered by a parathyroid adenoma,hereditary factors,parathyroid carcinoma,or renal osteodystrophy. Osteoclastic bone resorption releases minerals,including calcium,from the bone into the bloodstream,causing both elevated blood calcium levels,and the structural changes which weaken the bone. The symptoms of the disease are the consequences of both the general softening of the bones and the excess calcium in the blood,and include bone fractures,kidney stones,nausea,moth-eaten appearance in the bones,appetite loss,and weight loss.

McCune–Albright syndrome is a complex genetic disorder affecting the bone,skin and endocrine systems. It is a mosaic disease arising from somatic activating mutations in GNAS,which encodes the alpha-subunit of the Gs heterotrimeric G protein.

Fibroblast growth factor 23 (FGF-23) is a protein and member of the fibroblast growth factor (FGF) family which participates in the regulation of phosphate in plasma and vitamin D metabolism. In humans it is encoded by the FGF23 gene. FGF-23 decreases reabsorption of phosphate in the kidney. Mutations in FGF23 can lead to its increased activity,resulting in autosomal dominant hypophosphatemic rickets.

GNAS complex locus is a gene locus in humans. Its main product is the heterotrimeric G-protein alpha subunit Gs-α,a key component of G protein-coupled receptor-regulated adenylyl cyclase signal transduction pathways. GNAS stands for Guanine Nucleotide binding protein,Alpha Stimulating activity polypeptide.

X-linked hypophosphatemia (XLH) is an X-linked dominant form of rickets that differs from most cases of dietary deficiency rickets in that vitamin D supplementation does not cure it. It can cause bone deformity including short stature and genu varum (bow-leggedness). It is associated with a mutation in the PHEX gene sequence (Xp.22) and subsequent inactivity of the PHEX protein. PHEX mutations lead to an elevated circulating (systemic) level of the hormone FGF23 which results in renal phosphate wasting,and local elevations of the mineralization/calcification-inhibiting protein osteopontin in the extracellular matrix of bones and teeth. An inactivating mutation in the PHEX gene results in an increase in systemic circulating FGF23,and a decrease in the enzymatic activity of the PHEX enzyme which normally removes (degrades) mineralization-inhibiting osteopontin protein;in XLH,the decreased PHEX enzyme activity leads to an accumulation of inhibitory osteopontin locally in bones and teeth to block mineralization which,along with renal phosphate wasting,both cause osteomalacia and odontomalacia.

Fuller Albright was an American endocrinologist who made numerous contributions to his field,especially to the area of calcium metabolism. Albright made great strides and contributions to the understanding of disorders associated with calcium and phosphate abnormalities in the body. He also was a published author and in his books he detailed his findings.

Parathyroid hormone/parathyroid hormone-related peptide receptor,also known as parathyroid hormone 1 receptor (PTH1R),is a protein that in humans is encoded by the PTH1R gene. PTH1R functions as a receptor for parathyroid hormone (PTH) and for parathyroid hormone-related protein (PTHrP),also called parathyroid hormone-like hormone (PTHLH).

Cytochrome P450 family 24 subfamily A member 1 (abbreviated CYP24A1) is a member of the cytochrome P450 superfamily of enzymes encoded by the CYP24A1 gene. It is a mitochondrial monooxygenase which catalyzes reactions including 24-hydroxylation of calcitriol (1,25-dihydroxyvitamin D3). It has also been identified as vitamin D3 24-hydroxylase.(EC 1.14.15.16)
An endocrine bone disease is a bone disease associated with a disorder of the endocrine system. An example is osteitis fibrosa cystica.
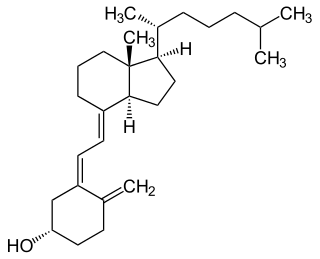
Vitamin D is a group of fat-soluble secosteroids responsible for increasing intestinal absorption of calcium,magnesium,and phosphate,along with numerous other biological functions. In humans,the most significant compounds within this group are vitamin D3 (cholecalciferol) and vitamin D2 (ergocalciferol).

Rajesh Vasantlal Thakker is May Professor of Medicine in the Nuffield Department of Clinical Medicine at the University of Oxford and a fellow of Somerville College,Oxford. Thakker is also a Consultant physician at the Churchill Hospital and the John Radcliffe Hospital,Principal investigator (PI) at the Oxford Centre for Diabetes,Endocrinology and Metabolism (OCDEM) and was Chairman of the NIHR/MRC Efficacy and Mechanism Evaluation (EME) Board until Spring 2016.
Walter L. Miller is an American endocrinologist and professor emeritus of pediatrics at the University of California,San Francisco (UCSF). Miller is expert in the field of human steroid biosynthesis and disorders of steroid metabolism. Over the past 40 years Miller's group at UCSF has described molecular basis of several metabolic disorders including,congenital adrenal hyperplasia,pseudo vitamin D dependent rickets,severe,recessive form of Ehlers-Danlos syndrome,17,20 lyase deficiency caused by CYP17A1 defects,P450scc deficiency caused by CYP11A1 defects,P450 oxidoreductase deficiency.
Phosphate diabetes is a rare,congenital,hereditary disorder associated with inadequate tubular reabsorption that affects the way the body processes and absorbs phosphate. Also named as X-linked dominant hypophosphatemic rickets (XLH),this disease is caused by a mutation in the X-linked PHEX gene,which encodes for a protein that regulates phosphate levels in the human body. phosphate is an essential mineral which plays a significant role in the formation and maintenance of bones and teeth,energy production and other important cellular processes. phosphate diabetes is a condition that falls under the category of tubulopathies,which refers to the pathologies of the renal tubules. The mutated PHEX gene causes pathological elevations in fibroblast growth factor 23 (FGF23),a hormone that regulates phosphate homeostasis by decreasing the reabsorption of phosphate in the kidneys.
References
- 1 2 3 "Michael Alan Levine | Faculty | Perelman School of Medicine at the University of Pennsylvania". www.med.upenn.edu.
- ↑ "Michael A. Levine, M.D." scholar.google.com.
- 1 2 Bilezikian, John P.; Marcus, Robert; Levine, Michael A., eds. (October 31, 2001). The parathyroids: basic and clinical concepts. Academic Press. ISBN 978-0-12-098651-4 – via Library of Congress ISBN.
- 1 2 "HGF | Lifetime Achievement Honorees". HGF.
- ↑ "The American Society for Clinical Investigation". the-asci.org.
- ↑ "Michael A. Levine, MD, FAAP, FACP, MACE , FACE". www.chop.edu. Children's Hospital of Philadelphia.
- ↑ "Michael A. Levine, M.D." scholar.google.com.
- ↑ Patten, Jennifer L.; Johns, Donald R.; Valle, David; Eil, Charles; Gruppuso, Philip A.; Steele, Gary; Smallwood, Philip M.; Levine, Michael A. (May 17, 1990). "Mutation in the Gene Encoding the Stimulatory G Protein of Adenylate Cyclase in Albright's Hereditary Osteodystrophy". New England Journal of Medicine. 322 (20): 1412–1419. doi:10.1056/NEJM199005173222002. PMID 2109828 – via pure.johnshopkins.edu.
- ↑ Schwindinger, W. F.; Francomano, C. A.; Levine, M. A. (June 1, 1992). "Identification of a mutation in the gene encoding the alpha subunit of the stimulatory G protein of adenylyl cyclase in McCune-Albright syndrome". Proceedings of the National Academy of Sciences of the United States of America. 89 (11): 5152–5156. Bibcode:1992PNAS...89.5152S. doi: 10.1073/pnas.89.11.5152 . PMC 49247 . PMID 1594625.
- ↑ Schnitzer, T.; Bone, H. G.; Crepaldi, G.; Adami, S.; McClung, M.; Kiel, D.; Felsenberg, D.; Recker, R. R.; Tonino, R. P.; Roux, C.; Pinchera, A.; Foldes, A. J.; Greenspan, S. L.; Levine, M. A.; Emkey, R.; Santora, A. C.; Kaur, A.; Thompson, D. E.; Yates, J.; Orloff, J. J. (February 29, 2000). "Therapeutic equivalence of alendronate 70 mg once-weekly and alendronate 10 mg daily in the treatment of osteoporosis. Alendronate Once-Weekly Study Group". Aging (Milan, Italy). 12 (1): 1–12. PMID 10746426 – via PubMed.
- ↑ Li, Q.; Brodsky, J. L.; Conlin, L. K.; Pawel, B.; Glatz, A. C.; Gafni, R. I.; Schurgers, L.; Uitto, J.; Hakonarson, H.; Deardorff, M. A.; Levine, M. A. (2013). "Mutations in the ABCC6 Gene as a Cause of Generalized Arterial Calcification of Infancy – Genotypic Overlap with Pseudoxanthoma Elasticum - PMC". The Journal of Investigative Dermatology. 134 (3): 658–665. doi:10.1038/jid.2013.370. PMC 3945730 . PMID 24008425.
- ↑ "Oncogenic osteomalacia-related gene 1".
- ↑ "Phosphatonin-related gene and methods of use thereof".
- ↑ "Frederic C. Bartter Award". American Society for Bone and Mineral Research. January 22, 2009.
- ↑ "Senior Investigator Award - Pediatric Endocrine Society". January 11, 2021.
- ↑ "Judson J. Van Wyk Prize - Pediatric Endocrine Society". June 25, 2020.
- ↑ The Parathyroids, Basic and Clinical Concepts. Academic Press. 9 September 2014. ISBN 978-0-12-397790-8.