
Lichenology is the branch of mycology that studies the lichens, symbiotic organisms made up of an intimate symbiotic association of a microscopic alga with a filamentous fungus. Lichens are chiefly characterized by this symbiosis.

Cladonia asahinae, the pixie cup lichen or Asahina's cup lichen, is a species of cup lichen in the family Cladoniaceae. C. asahinae occurs in Europe, North America, southern South America, and the Antarctic. It typically grows in high moisture environments in soil rich in humus or on dead wood.

Punctelia is a genus of foliose lichens belonging to the large family Parmeliaceae. The genus, which contains about 50 species, was segregated from genus Parmelia in 1982. Characteristics that define Punctelia include the presence of hook-like to thread-like conidia, simple rhizines, and point-like pseudocyphellae. It is this last feature that is alluded to in the vernacular names speckled shield lichens or speckleback lichens.
A spot test in lichenology is a spot analysis used to help identify lichens. It is performed by placing a drop of a chemical reagent on different parts of the lichen and noting the colour change associated with application of the chemical. The tests are routinely encountered in dichotomous keys for lichen species, and they take advantage of the wide array of lichen products produced by lichens and their uniqueness among taxa. As such, spot tests reveal the presence or absence of chemicals in various parts of a lichen. They were first proposed as a method to help identify species by the Finnish lichenologist William Nylander in 1866.

Yasuhiko Asahina was a Japanese chemist and lichenologist.

Punctelia rudecta, commonly known as the rough speckled shield or the speckleback lichen, is a North American species of foliose lichen in the family Parmeliaceae. This species can be readily identified by the light color of the thallus underside, the relatively large lobes at the edges of the thallus, and the tiny white pores present on the top of the thallus that are characteristic of the genus Punctelia. The lichen is quite abundant and widespread in the eastern and southeastern United States, although it also occurs in Canada and northern Mexico, but is less common in these regions. The lichen usually grows on bark, and less commonly on shaded rocks. There are several lookalike Punctelia species; these can often be distinguished from P. rudecta by differences in distribution or in the nature of the reproductive structures present on the thallus.

Punctelia caseana is a species of foliose lichen in the family Parmeliaceae. Its range covers eastern North America, extending south to central and northern Mexico, where it grows on the bark of many species of hardwood and conifer trees.
Punctelia crispa is a species of foliose lichen in the family Parmeliaceae. Found in Brazil, it was described as a new species in 2009 by lichenologists Marcello Marcelli, Patrícia Jungbluth, and John Alan Elix. The holotype specimen was collected in the Botujuru quarter of Campo Limpo Paulista municipality in São Paulo State. There it was found in a transition zone between a mesophyllous forest and a cerrado forest, growing on a tree trunk. It has a greyish thallus loosely attached to its substrate, measuring up to 10 cm (4 in) in diameter. Soredia on the thallus surface have a coarse texture, and are dense so that it gives areas near the thallus centre a "crispate" appearance, a feature for which the species is named. The medulla is white, while the lower surface of the thallus is pale brown, becoming darker near the centre. The lichen contains atranorin as a minor substance, gyrophoric acid as a major substance, and trace amounts of lecanoric acid.

Constipatic acid is a fatty acid found in several lichen species. It was isolated, identified, and named by Douglas Chester and John Alan Elix in a 1979 publication. The compound was extracted from the Australian leafy lichen called Xanthoparmelia constipata, which was collected on schist boulders west of Springton, South Australia. The related compounds protoconstipatic acid and dehydroconstipatic acid were also reported concurrently. Syo Kurokawa and Rex Filson had previously detected the compounds using thin-layer chromatography when they formally described the lichen as a new species in 1975, but had not characterised them chemically.

Punctelia hypoleucites, commonly known as the southwestern speckled shield lichen, is a species of foliose (leafy) lichen in the family Parmeliaceae. First formally described by Finnish botanist William Nylander as a species of Parmelia, it was transferred to the genus Punctelia in 1982. The lichen is found in Africa, North America, and South America, where it grows on the bark of both hardwood and coniferous trees. Its greenish-grey thallus is covered with tiny white pseudocyphellae – minute holes in the thallus surface that facilitate gas exchange. Some macroscopic features that help distinguish this species from other related members of the genus include the presence and the structure of the apothecia, the absence of asexual surface propagules, and the light brown color of the thallus undersurface. Chemically, the presence of lecanoric acid in the medulla and atranorin in the cortex help distinguish it from lookalikes.

Punctelia perreticulata is a widely distributed species of foliose lichen in the family Parmeliaceae. It occurs in Mediterranean Europe and Russia, North America, South America, Australia, and New Zealand, where it grows on rocks, bark, or wood. Its main distinguishing features are its thallus surface, marked with many shallow depressions, grooves, or pits, and sorediate pseudocyphellae. The lower side of the thallus is ivory to tan towards the centre and the major secondary metabolite in the medulla is lecanoric acid. A lookalike species with which it has been historically confused is Punctelia subrudecta; this lichen can be distinguished from Punctelia perreticulata by the texture of the thallus surface, or, more reliably, by the length of its conidia.

Punctelia borreri is a species of foliose lichen in the family Parmeliaceae. It is a common and widely distributed species, occurring in tropical, subtropical, and temperate regions of Africa, Asia, Europe, North America, Oceania, and South America. The lichen typically grows on bark of deciduous trees, and less commonly on rock. Some European countries have reported increases in the geographic range or regional frequency of the lichen in recent decades, attributed alternatively to a reduction of atmospheric sulphur dioxide levels or an increase in temperatures resulting from climate change.

Salazinic acid is a depsidone with a lactone ring. It is found in some lichens, and is especially prevalent in Parmotrema and Bulbothrix, where its presence or absence is often used to help classify species in those genera.

Lichexanthone is an organic compound in the structural class of chemicals known as xanthones. Lichexanthone was first isolated and identified by Japanese chemists from a species of leafy lichen in the 1940s. The compound is known to occur in many lichens, and it is important in the taxonomy of species in several genera, such as Pertusaria and Pyxine. More than a dozen lichen species have a variation of the word lichexanthone incorporated as part of their binomial name. The presence of lichexanthone in lichens causes them to fluoresce a greenish-yellow colour under long-wavelength UV light; this feature is used to help identify some species. Lichexanthone is also found in several plants, and some species of fungi that do not form lichens.
Lichen products, also known as lichen substances, are organic compounds produced by a lichen. Specifically, they are secondary metabolites. Lichen products are represented in several different chemical classes, including terpenoids, orcinol derivatives, chromones, xanthones, depsides, and depsidones. Over 800 lichen products of known chemical structure have been reported in the scientific literature, and most of these compounds are exclusively found in lichens. Examples of lichen products include usnic acid, atranorin, lichexanthone, salazinic acid, and isolichenan, an α-glucan. Many lichen products have biological activity, and research into these effects is ongoing.

Barbatic acid is an organic compound that is made by some lichens. It is in the structural class known as depsides. It is particularly common in the genera Usnea and Cladonia.

Siegfried Huneck was a German chemist and lichenologist. Much of his scientific career was hampered by the political situation in the former German Democratic Republic. He rejected pursuing a career in academia, and instead ended up working at the Leibniz Institute of Plant Biochemistry, a public research institute, from 1969 until his retirement in 1993. Despite his relative isolation and restricted freedoms in East Germany, Huneck had numerous professional contacts both in Germany and abroad, and was a highly published scholar. Many of his more than 400 scientific publications dealt with the chemistry of lichen products. He was awarded the Acharius Medal for lifetime achievements in lichenology in 1996.
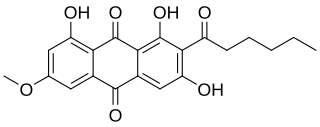
Solorinic acid is an anthraquinone pigment found in the leafy lichen Solorina crocea. It is responsible for the strong orange colour of the medulla and the underside of the thallus in that species. In its purified crystalline form, it exists as orange-red crystals with a melting point of 201 °C (394 °F).
Opegrapha ramisorediata is a rare species of corticolous (bark-dwelling), crustose lichen in the family Opegraphaceae. Known to occur only in northeastern Brazil, it was described as a new species in 2017. It is characterised by a thin, pale greenish-mauve thallus.

Confluentic acid is an organic compound belonging to the chemical class known as depsides. It serves as a secondary metabolite in certain lichens and plays a role in distinguishing closely related species within the genus Porpidia. In 1899, Friedrich Wilhelm Zopf isolated a compound from Lecidea confluens, which he initially named confluentin and noted for its melting point of 147–148 °C. This substance demonstrated the ability to turn litmus paper red and, when interacting with alkali, decomposed into carbon dioxide and phenol-like compounds. Zopf subsequently revised the chemical formula and melting point of the compound. Siegfried Huneck renamed it confluentinic acid in 1962, characterising it as optically inactive, with distinct colour reactions and solubility properties, and determined its molecular formula as C28H36O8.















