
In geology and mineralogy, a mineral or mineral species is, broadly speaking, a solid substance with a fairly well-defined chemical composition and a specific crystal structure that occurs naturally in pure form.

A mafic mineral or rock is a silicate mineral or igneous rock rich in magnesium and iron. Most mafic minerals are dark in color, and common rock-forming mafic minerals include olivine, pyroxene, amphibole, and biotite. Common mafic rocks include basalt, diabase and gabbro. Mafic rocks often also contain calcium-rich varieties of plagioclase feldspar. Mafic materials can also be described as ferromagnesian.

Amphibole is a group of inosilicate minerals, forming prism or needlelike crystals, composed of double chain SiO
4 tetrahedra, linked at the vertices and generally containing ions of iron and/or magnesium in their structures. Its IMA symbol is Amp. Amphiboles can be green, black, colorless, white, yellow, blue, or brown. The International Mineralogical Association currently classifies amphiboles as a mineral supergroup, within which are two groups and several subgroups.

Amphibolite is a metamorphic rock that contains amphibole, especially hornblende and actinolite, as well as plagioclase feldspar, but with little or no quartz. It is typically dark-colored and dense, with a weakly foliated or schistose (flaky) structure. The small flakes of black and white in the rock often give it a salt-and-pepper appearance.

Metasomatism is the chemical alteration of a rock by hydrothermal and other fluids. It is traditionally defined as metamorphism which involves a change in the chemical composition, excluding volatile components. It is the replacement of one rock by another of different mineralogical and chemical composition. The minerals which compose the rocks are dissolved and new mineral formations are deposited in their place. Dissolution and deposition occur simultaneously and the rock remains solid.

The scapolites are a group of rock-forming silicate minerals composed of aluminium, calcium, and sodium silicate with chlorine, carbonate and sulfate. The two endmembers are meionite and marialite. Silvialite (Ca,Na)4Al6Si6O24(SO4,CO3) is also a recognized member of the group.

Silicate minerals are rock-forming minerals made up of silicate groups. They are the largest and most important class of minerals and make up approximately 90 percent of Earth's crust.

Granulites are a class of high-grade metamorphic rocks of the granulite facies that have experienced high-temperature and moderate-pressure metamorphism. They are medium to coarse–grained and mainly composed of feldspars sometimes associated with quartz and anhydrous ferromagnesian minerals, with granoblastic texture and gneissose to massive structure. They are of particular interest to geologists because many granulites represent samples of the deep continental crust. Some granulites experienced decompression from deep in the Earth to shallower crustal levels at high temperature; others cooled while remaining at depth in the Earth.

Hornfels is the group name for a set of contact metamorphic rocks that have been baked and hardened by the heat of intrusive igneous masses and have been rendered massive, hard, splintery, and in some cases exceedingly tough and durable. These properties are caused by fine grained non-aligned crystals with platy or prismatic habits, characteristic of metamorphism at high temperature but without accompanying deformation. The term is derived from the German word Hornfels, meaning "hornstone", because of its exceptional toughness and texture both reminiscent of animal horns. These rocks were referred to by miners in northern England as whetstones.

The chlorites are the group of phyllosilicate minerals common in low-grade metamorphic rocks and in altered igneous rocks. Greenschist, formed by metamorphism of basalt or other low-silica volcanic rock, typically contains significant amounts of chlorite.

Greenschists are metamorphic rocks that formed under the lowest temperatures and pressures usually produced by regional metamorphism, typically 300–450 °C (570–840 °F) and 2–10 kilobars (29,000–145,000 psi). Greenschists commonly have an abundance of green minerals such as chlorite, serpentine, and epidote, and platy minerals such as muscovite and platy serpentine. The platiness gives the rock schistosity. Other common minerals include quartz, orthoclase, talc, carbonate minerals and amphibole (actinolite).

Litchfieldite is a rare igneous rock. It is a coarse-grained, foliated variety of nepheline syenite, sometimes called nepheline syenite gneiss or gneissic nepeheline syenite. Litchfieldite is composed of two varieties of feldspar, with nepheline, sodalite, cancrinite and calcite. The mafic minerals, when present, are magnetite and an iron-rich variety of biotite (lepidomelane).
Talc carbonates are a suite of rock and mineral compositions found in metamorphosed ultramafic rocks.
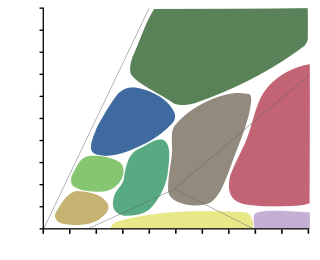
A metamorphic facies is a set of mineral assemblages in metamorphic rocks formed under similar pressures and temperatures. The assemblage is typical of what is formed in conditions corresponding to an area on the two dimensional graph of temperature vs. pressure. Rocks which contain certain minerals can therefore be linked to certain tectonic settings, times and places in the geological history of the area. The boundaries between facies are wide because they are gradational and approximate. The area on the graph corresponding to rock formation at the lowest values of temperature and pressure is the range of formation of sedimentary rocks, as opposed to metamorphic rocks, in a process called diagenesis.
This glossary of geology is a list of definitions of terms and concepts relevant to geology, its sub-disciplines, and related fields. For other terms related to the Earth sciences, see Glossary of geography terms.
The Piégut-Pluviers Granodiorite is situated at the northwestern edge of the Variscan Massif Central in France. Its cooling age has been determined as 325 ± 14 million years BP.
The Tabataud Quarry is situated in the northwestern French Massif Central. The quarry used to be mined for its granodiorite.

A subduction zone is a region of the Earth's crust where one tectonic plate moves under another tectonic plate; oceanic crust gets recycled back into the mantle and continental crust gets produced by the formation of arc magmas. Arc magmas account for more than 20% of terrestrially produced magmas and are produced by the dehydration of minerals within the subducting slab as it descends into the mantle and are accreted onto the base of the overriding continental plate. Subduction zones host a unique variety of rock types formed by the high-pressure, low-temperature conditions a subducting slab encounters during its descent. The metamorphic conditions the slab passes through in this process generates and alters water bearing (hydrous) mineral phases, releasing water into the mantle. This water lowers the melting point of mantle rock, initiating melting. Understanding the timing and conditions in which these dehydration reactions occur, is key to interpreting mantle melting, volcanic arc magmatism, and the formation of continental crust.

The Siilinjärvi carbonatite complex is located in central Finland close to the city of Kuopio. It is named after the nearby town of Siilinjärvi, located approximately 5 km west of the southern extension of the complex. Siilinjärvi is the second largest carbonatite complex in Finland after the Sokli formation, and one of the oldest carbonatites on Earth at 2610±4 Ma. The carbonatite complex consists of a roughly 16 km long steeply dipping lenticular body surrounded by granite gneiss. The maximum width of the body is 1.5 km and the surface area is 14.7 km2. The complex was discovered in 1950 by the Geological Survey of Finland with help of local mineral collectors. The exploration drilling began in 1958 by Lohjan Kalkkitehdas Oy. Typpi Oy continued drilling between years 1964 and 1967, and Apatiitti Oy drilled from 1967 to 1968. After the drillings, the laboratory and pilot plant work were made. The mine was opened by Kemira Oyj in 1979 as an open pit. The operation was sold to Yara in 2007.














