
The mevalonate pathway, also known as the isoprenoid pathway or HMG-CoA reductase pathway is an essential metabolic pathway present in eukaryotes, archaea, and some bacteria. The pathway produces two five-carbon building blocks called isopentenyl pyrophosphate (IPP) and dimethylallyl pyrophosphate (DMAPP), which are used to make isoprenoids, a diverse class of over 30,000 biomolecules such as cholesterol, vitamin K, coenzyme Q10, and all steroid hormones.
Simvastatin, sold under the brand name Zocor among others, is a statin, a type of lipid-lowering medication. It is used along with exercise, diet, and weight loss to decrease elevated lipid levels. It is also used to decrease the risk of heart problems in those at high risk. It is taken by mouth.

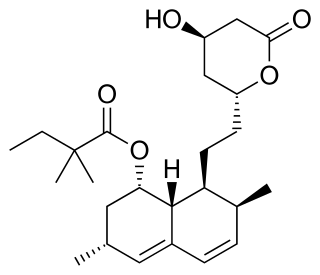
Pravastatin, sold under the brand name Pravachol among others, is a statin medication, used for preventing cardiovascular disease in those at high risk and treating abnormal lipids. It should be used together with diet changes, exercise, and weight loss. It is taken by mouth.

Lovastatin, sold under the brand name Mevacor among others, is a statin medication, to treat high blood cholesterol and reduce the risk of cardiovascular disease. Its use is recommended together with lifestyle changes. It is taken by mouth.

Cerivastatin is a synthetic member of the class of statins used to lower cholesterol and prevent cardiovascular disease. It was marketed by the pharmaceutical company Bayer A.G. in the late 1990s, competing with Pfizer's highly successful atorvastatin (Lipitor). Cerivastatin was voluntarily withdrawn from the market worldwide in 2001, due to reports of fatal rhabdomyolysis.

HMG-CoA reductase is the rate-controlling enzyme of the mevalonate pathway, the metabolic pathway that produces cholesterol and other isoprenoids. HMGCR catalyzes the conversion of HMG-CoA to mevalonic acid, a necessary step in the biosynthesis of cholesterol. Normally in mammalian cells this enzyme is competitively suppressed so that its effect is controlled. This enzyme is the target of the widely available cholesterol-lowering drugs known collectively as the statins, which help treat dyslipidemia.

Prenylation is the addition of hydrophobic molecules to a protein or a biomolecule. It is usually assumed that prenyl groups (3-methylbut-2-en-1-yl) facilitate attachment to cell membranes, similar to lipid anchors like the GPI anchor, though direct evidence of this has not been observed. Prenyl groups have been shown to be important for protein–protein binding through specialized prenyl-binding domains.

Smith–Lemli–Opitz syndrome is an inborn error of cholesterol synthesis. It is an autosomal recessive, multiple malformation syndrome caused by a mutation in the enzyme 7-Dehydrocholesterol reductase encoded by the DHCR7 gene. It causes a broad spectrum of effects, ranging from mild intellectual disability and behavioural problems to lethal malformations.

Mevastatin is a hypolipidemic agent that belongs to the statins class.

Mevalonic acid (MVA) is a key organic compound in biochemistry; the name is a contraction of dihydroxymethylvalerolactone. The carboxylate anion of mevalonic acid, which is the predominant form in biological environments, is known as mevalonate and is of major pharmaceutical importance. Drugs like statins stop the production of mevalonate by inhibiting HMG-CoA reductase.

Pitavastatin is a member of the blood cholesterol lowering medication class of statins.

Mexazolam is a drug which is a benzodiazepine derivative. Mexazolam has been trialed for anxiety and was found to be effective in alleviating anxiety at one week follow-up. Mexazolam is metabolised via the CYP3A4 pathway. HMG-CoA reductase inhibitors including simvastatin, simvastatin acid, lovastatin, fluvastatin, atorvastatin and cerivastatin inhibit the metabolism of mexazolam, but not the HMG-CoA reductase inhibitor pravastatin. Its principal active metabolites are chlordesmethyldiazepam and chloroxazepam.

Squalene synthase (SQS) or farnesyl-diphosphate:farnesyl-diphosphate farnesyl transferase is an enzyme localized to the membrane of the endoplasmic reticulum. SQS participates in the isoprenoid biosynthetic pathway, catalyzing a two-step reaction in which two identical molecules of farnesyl pyrophosphate (FPP) are converted into squalene, with the consumption of NADPH. Catalysis by SQS is the first committed step in sterol synthesis, since the squalene produced is converted exclusively into various sterols, such as cholesterol, via a complex, multi-step pathway. SQS belongs to squalene/phytoene synthase family of proteins.
The discovery of HMG-CoA (3-hydroxy-3-methylglutaryl-CoA) reductase inhibitors, called statins, was a breakthrough in the prevention of hypercholesterolemia and related diseases. Hypercholesterolemia is considered to be one of the major risk factors for atherosclerosis which often leads to cardiovascular, cerebrovascular and peripheral vascular diseases. The statins inhibit cholesterol synthesis in the body and that leads to reduction in blood cholesterol levels, which is thought to reduce the risk of atherosclerosis and diseases caused by it.

Garcinia dulcis is a tropical fruit tree native to the Philippines, Java, Lesser Sunda Islands, eastern Indonesia, and Papua New Guinea. It was domesticated early and spread inland into mainland Asia. It is commonly known as mundu or munu in Indonesia and Malaysia, baniti or taklang-anak in the Philippines, and maphuut or ma phut in Thailand.
Bruce D. Roth is an American organic and medicinal chemist who trained at Iowa State University and the University of Rochester, and, at the age of 32, discovered atorvastatin, the statin-class drug sold as Lipitor that would become the largest-selling drug in pharmaceutical history. His honours include being named a 2008 Hero of Chemistry by the American Chemical Society, and being chosen as the Perkin Medal awardee, the highest honour given in the U.S. chemical industry, by the Society of Chemical Industry, American section in 2013.
In pharmacology, pleiotropy includes all of a drug's actions other than those for which the agent was specifically developed. It may include adverse effects which are detrimental ones, but is often used to denote additional beneficial effects.

Sitagliptin/simvastatin, sold under the brand name Juvisync, is a fixed-dose combination anti-diabetic medication used to treat type 2 diabetes and hypercholesterolemia. It contains sitagliptin and simvastatin. Sitagliptin is a dipeptidyl peptidase-4 inhibitor and simvastatin is an HMG-CoA reductase inhibitor. These two disorders commonly occur in people at the same time, and have been typically treated with administration of these medications separately. The combination was approved in 2011, and sold under the brand name Juvisync by Merck. Juvisync was later removed from the market in 2013, due to business reasons.
A steroidogenesis inhibitor, also known as a steroid biosynthesis inhibitor, is a type of drug which inhibits one or more of the enzymes that are involved in the process of steroidogenesis, the biosynthesis of endogenous steroids and steroid hormones. They may inhibit the production of cholesterol and other sterols, sex steroids such as androgens, estrogens, and progestogens, corticosteroids such as glucocorticoids and mineralocorticoids, and neurosteroids. They are used in the treatment of a variety of medical conditions that depend on endogenous steroids.
Garcinia binucao is a species of flowering plant in the Clusiaceae family. It is commonly known as binukaw or batuan, is a species of Garcinia endemic to the Philippines. It is not cultivated, though its edible fruits are harvested from the wild for use as a souring agent in some Filipino dishes.















