Related Research Articles
In chemical analysis, chromatography is a laboratory technique for the separation of a mixture into its components. The mixture is dissolved in a fluid solvent called the mobile phase, which carries it through a system on which a material called the stationary phase is fixed. Because the different constituents of the mixture tend to have different affinities for the stationary phase and are retained for different lengths of time depending on their interactions with its surface sites, the constituents travel at different apparent velocities in the mobile fluid, causing them to separate. The separation is based on the differential partitioning between the mobile and the stationary phases. Subtle differences in a compound's partition coefficient result in differential retention on the stationary phase and thus affect the separation.

Gel electrophoresis is a method for separation and analysis of biomacromolecules and their fragments, based on their size and charge. It is used in clinical chemistry to separate proteins by charge or size and in biochemistry and molecular biology to separate a mixed population of DNA and RNA fragments by length, to estimate the size of DNA and RNA fragments or to separate proteins by charge.
The isoelectric point (pI, pH(I), IEP), is the pH at which a molecule carries no net electrical charge or is electrically neutral in the statistical mean. The standard nomenclature to represent the isoelectric point is pH(I). However, pI is also used. For brevity, this article uses pI. The net charge on the molecule is affected by pH of its surrounding environment and can become more positively or negatively charged due to the gain or loss, respectively, of protons (H+).

In chemistry, electrophoresis is the motion of charged dispersed particles or dissolved charged molecules relative to a fluid under the influence of a spatially uniform electric field. As a rule, these are zwitterions. Electrophoresis of positively charged particles or molecules (cations) is sometimes called cataphoresis, while electrophoresis of negatively charged particles or molecules (anions) is sometimes called anaphoresis.
Protein purification is a series of processes intended to isolate one or a few proteins from a complex mixture, usually cells, tissues or whole organisms. Protein purification is vital for the specification of the function, structure and interactions of the protein of interest. The purification process may separate the protein and non-protein parts of the mixture, and finally separate the desired protein from all other proteins. Ideally, to study a protein of interest, it must be separated from other components of the cell so that contaminants will not interfere in the examination of the protein of interest's structure and function. Separation of one protein from all others is typically the most laborious aspect of protein purification. Separation steps usually exploit differences in protein size, physico-chemical properties, binding affinity and biological activity. The pure result may be termed protein isolate.

Protein electrophoresis is a method for analysing the proteins in a fluid or an extract. The electrophoresis may be performed with a small volume of sample in a number of alternative ways with or without a supporting medium, namely agarose or polyacrylamide. Variants of gel electrophoresis include SDS-PAGE, free-flow electrophoresis, electrofocusing, isotachophoresis, affinity electrophoresis, immunoelectrophoresis, counterelectrophoresis, and capillary electrophoresis. Each variant has many subtypes with individual advantages and limitations. Gel electrophoresis is often performed in combination with electroblotting or immunoblotting to give additional information about a specific protein.

Isoelectric focusing (IEF), also known as electrofocusing, is a technique for separating different molecules by differences in their isoelectric point (pI). It is a type of zone electrophoresis usually performed on proteins in a gel that takes advantage of the fact that overall charge on the molecule of interest is a function of the pH of its surroundings.
Capillary electrophoresis (CE) is a family of electrokinetic separation methods performed in submillimeter diameter capillaries and in micro- and nanofluidic channels. Very often, CE refers to capillary zone electrophoresis (CZE), but other electrophoretic techniques including capillary gel electrophoresis (CGE), capillary isoelectric focusing (CIEF), capillary isotachophoresis and micellar electrokinetic chromatography (MEKC) belong also to this class of methods. In CE methods, analytes migrate through electrolyte solutions under the influence of an electric field. Analytes can be separated according to ionic mobility and/or partitioning into an alternate phase via non-covalent interactions. Additionally, analytes may be concentrated or "focused" by means of gradients in conductivity and pH.

Arne Wilhelm Kaurin Tiselius was a Swedish biochemist who won the Nobel Prize in Chemistry in 1948 "for his research on electrophoresis and adsorption analysis, especially for his discoveries concerning the complex nature of the serum proteins."

Coacervate is an aqueous phase rich in macromolecules such as synthetic polymers, proteins or nucleic acids. It forms through liquid-liquid phase separation (LLPS), leading to a dense phase in thermodynamic equilibrium with a dilute phase. The dispersed droplets of dense phase are also called coacervates, micro-coacervates or coacervate droplets. These structures draw a lot of interest because they form spontaneously from aqueous mixtures and provide stable compartmentalization without the need of a membrane.
QPNC-PAGE, or QuantitativePreparativeNativeContinuousPolyacrylamideGel Electrophoresis, is a bioanalytical, one-dimensional, high-resolution and high-precision technique applied in biochemistry and bioinorganic chemistry to separate proteins quantitatively by isoelectric point and by continuous elution from a gel column. This standardized variant of native gel electrophoresis and subset of preparative polyacrylamide gel electrophoresis is used by biologists to isolate macromolecules in solution, for example, active or native metalloproteins in biological samples or properly and improperly folded metal cofactor-containing proteins or protein isoforms in complex protein mixtures.
DNA separation by silica adsorption is a method of DNA separation that is based on DNA molecules binding to silica surfaces in the presence of certain salts and under certain pH conditions.
Acid–base extraction is a subclass of liquid–liquid extractions and involves the separation of chemical species from other acidic or basic compounds. It is typically performed during the work-up step following a chemical synthesis to purify crude compounds and results in the product being largely free of acidic or basic impurities. A separatory funnel is commonly used to perform an acid-base extraction.
Electrophoretic light scattering is based on dynamic light scattering. The frequency shift or phase shift of an incident laser beam depends on the dispersed particles mobility. With dynamic light scattering, Brownian motion causes particle motion. With electrophoretic light scattering, oscillating electric field performs this function.

Harvey Akio Itano was an American biochemist best known for his work on the molecular basis of sickle cell anemia and other diseases. In collaboration with Linus Pauling, Itano used electrophoresis to demonstrate the difference between normal hemoglobin and sickle cell hemoglobin; their 1949 paper "Sickle Cell Anemia, a Molecular Disease" was a landmark in both molecular medicine and protein electrophoresis, though the use of electrophoresis to separate hemoglobin variants had been pioneered by Maud Menten and collaborators some years earlier.
"Sickle Cell Anemia, a Molecular Disease" is a 1949 scientific paper by Linus Pauling, Harvey A. Itano, Seymour J. Singer and Ibert C. Wells that established sickle-cell anemia as a genetic disease in which affected individuals have a different form of the metalloprotein hemoglobin in their blood. The paper, published in the November 25, 1949 issue of Science, reports a difference in electrophoretic mobility between hemoglobin from healthy individuals and those with sickle-cell anemia, with those with sickle cell trait having a mixture of the two types. The paper suggests that the difference in electrophoretic mobility is probably due to a different number of ionizable amino acid residues in the protein portion of hemoglobin, and that this change in molecular structure is responsible for the sickling process. It also reports the genetic basis for the disease, consistent with the simultaneous genealogical study by James V. Neel: those with sickle-cell anemia are homozygous for the disease gene, while heterozygous individuals exhibit the usually asymptomatic condition of sickle cell trait.

Affinity electrophoresis is a general name for many analytical methods used in biochemistry and biotechnology. Both qualitative and quantitative information may be obtained through affinity electrophoresis. Cross electrophoresis, the first affinity electrophoresis method, was created by Nakamura et al. Enzyme-substrate complexes have been detected using cross electrophoresis. The methods include the so-called electrophoretic mobility shift assay, charge shift electrophoresis and affinity capillary electrophoresis. The methods are based on changes in the electrophoretic pattern of molecules through biospecific interaction or complex formation. The interaction or binding of a molecule, charged or uncharged, will normally change the electrophoretic properties of a molecule. Membrane proteins may be identified by a shift in mobility induced by a charged detergent. Nucleic acids or nucleic acid fragments may be characterized by their affinity to other molecules. The methods have been used for estimation of binding constants, as for instance in lectin affinity electrophoresis or characterization of molecules with specific features like glycan content or ligand binding. For enzymes and other ligand-binding proteins, one-dimensional electrophoresis similar to counter electrophoresis or to "rocket immunoelectrophoresis", affinity electrophoresis may be used as an alternative quantification of the protein. Some of the methods are similar to affinity chromatography by use of immobilized ligands.
The history of electrophoresis for molecular separation and chemical analysis began with the work of Arne Tiselius in 1931, while new separation processes and chemical analysis techniques based on electrophoresis continue to be developed in the 21st century. Tiselius, with support from the Rockefeller Foundation, developed the "Tiselius apparatus" for moving boundary electrophoresis, which was described in 1937 in the well-known paper "A New Apparatus for Electrophoretic Analysis of Colloidal Mixtures". The method spread slowly until the advent of effective zone electrophoresis methods in the 1940s and 1950s, which used filter paper or gels as supporting media. By the 1960s, increasingly sophisticated gel electrophoresis methods made it possible to separate biological molecules based on minute physical and chemical differences, helping to drive the rise of molecular biology. Gel electrophoresis and related techniques became the basis for a wide range of biochemical methods, such as protein fingerprinting, Southern blot, other blotting procedures, DNA sequencing, and many more.
Lattice density functional theory (LDFT) is a statistical theory used in physics and thermodynamics to model a variety of physical phenomena with simple lattice equations.
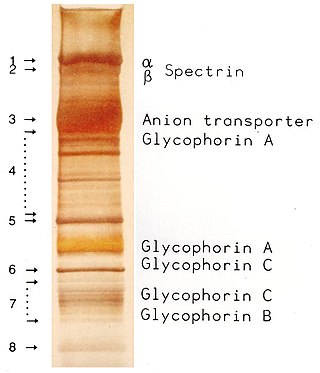
SDS-PAGE is a discontinuous electrophoretic system developed by Ulrich K. Laemmli which is commonly used as a method to separate proteins with molecular masses between 5 and 250 kDa. The combined use of sodium dodecyl sulfate and polyacrylamide gel eliminates the influence of structure and charge, and proteins are separated by differences in their size. At least up to 2012, the publication describing it was the most frequently cited paper by a single author, and the second most cited overall.
References
- ↑ Marmur, Abraham (1982). "Moving-boundary electrophoresis: Theory and interpretation of "anomalies"". Journal of Colloid and Interface Science. 85 (2): 556–565. Bibcode:1982JCIS...85..556M. doi:10.1016/0021-9797(82)90022-4. ISSN 0021-9797.
- ↑ Stern, Kurt G. (1939). "THE MOVING BOUNDARY METHOD FOR STUDYING Electrophoresis". Annals of the New York Academy of Sciences. 39 (1): 147–186. Bibcode:1939NYASA..39..147S. doi:10.1111/j.1749-6632.1939.tb55374.x. ISSN 0077-8923. S2CID 85079793.
- ↑ Arne Tiselius (1930). The Moving Boundary Method of Studying the Electrophoresis of Proteins. Inaugural Dissertation Upsala.
- ↑ Reiner Westermeier (21 March 2001). Electrophoresis in Practice. Wiley. ISBN 978-3-527-30300-7.