Microfluidics refers to a system that manipulates a small amount of fluids ( using small channels with sizes ten to hundreds micrometres. It is a multidisciplinary field that involves molecular analysis, biodefence, molecular biology, and microelectronics. It has practical applications in the design of systems that process low volumes of fluids to achieve multiplexing, automation, and high-throughput screening. Microfluidics emerged in the beginning of the 1980s and is used in the development of inkjet printheads, DNA chips, lab-on-a-chip technology, micro-propulsion, and micro-thermal technologies.

The enzyme-linked immunosorbent assay (ELISA) is a commonly used analytical biochemistry assay, first described by Eva Engvall and Peter Perlmann in 1971. The assay uses a solid-phase type of enzyme immunoassay (EIA) to detect the presence of a ligand in a liquid sample using antibodies directed against the protein to be measured. ELISA has been used as a diagnostic tool in medicine, plant pathology, and biotechnology, as well as a quality control check in various industries.

Digital microfluidics (DMF) is a platform for lab-on-a-chip systems that is based upon the manipulation of microdroplets. Droplets are dispensed, moved, stored, mixed, reacted, or analyzed on a platform with a set of insulated electrodes. Digital microfluidics can be used together with analytical analysis procedures such as mass spectrometry, colorimetry, electrochemical, and electrochemiluminescense.
A biosensor is an analytical device, used for the detection of a chemical substance, that combines a biological component with a physicochemical detector. The sensitive biological element, e.g. tissue, microorganisms, organelles, cell receptors, enzymes, antibodies, nucleic acids, etc., is a biologically derived material or biomimetic component that interacts with, binds with, or recognizes the analyte under study. The biologically sensitive elements can also be created by biological engineering. The transducer or the detector element, which transforms one signal into another one, works in a physicochemical way: optical, piezoelectric, electrochemical, electrochemiluminescence etc., resulting from the interaction of the analyte with the biological element, to easily measure and quantify. The biosensor reader device connects with the associated electronics or signal processors that are primarily responsible for the display of the results in a user-friendly way. This sometimes accounts for the most expensive part of the sensor device, however it is possible to generate a user friendly display that includes transducer and sensitive element. The readers are usually custom-designed and manufactured to suit the different working principles of biosensors.
An assay is an investigative (analytic) procedure in laboratory medicine, mining, pharmacology, environmental biology and molecular biology for qualitatively assessing or quantitatively measuring the presence, amount, or functional activity of a target entity. The measured entity is often called the analyte, the measurand, or the target of the assay. The analyte can be a drug, biochemical substance, chemical element or compound, or cell in an organism or organic sample. An assay usually aims to measure an analyte's intensive property and express it in the relevant measurement unit.

In molecular biology, biochips are engineered substrates that can host large numbers of simultaneous biochemical reactions. One of the goals of biochip technology is to efficiently screen large numbers of biological analytes, with potential applications ranging from disease diagnosis to detection of bioterrorism agents. For example, digital microfluidic biochips are under investigation for applications in biomedical fields. In a digital microfluidic biochip, a group of (adjacent) cells in the microfluidic array can be configured to work as storage, functional operations, as well as for transporting fluid droplets dynamically.
A lab-on-a-chip (LOC) is a device that integrates one or several laboratory functions on a single integrated circuit of only millimeters to a few square centimeters to achieve automation and high-throughput screening. LOCs can handle extremely small fluid volumes down to less than pico-liters. Lab-on-a-chip devices are a subset of microelectromechanical systems (MEMS) devices and sometimes called "micro total analysis systems" (µTAS). LOCs may use microfluidics, the physics, manipulation and study of minute amounts of fluids. However, strictly regarded "lab-on-a-chip" indicates generally the scaling of single or multiple lab processes down to chip-format, whereas "µTAS" is dedicated to the integration of the total sequence of lab processes to perform chemical analysis.
An immunoassay (IA) is a biochemical test that measures the presence or concentration of a macromolecule or a small molecule in a solution through the use of an antibody (usually) or an antigen (sometimes). The molecule detected by the immunoassay is often referred to as an "analyte" and is in many cases a protein, although it may be other kinds of molecules, of different sizes and types, as long as the proper antibodies that have the required properties for the assay are developed. Analytes in biological liquids such as serum or urine are frequently measured using immunoassays for medical and research purposes.
Point-of-care testing (POCT), also called near-patient testing or bedside testing, is defined as medical diagnostic testing at or near the point of care—that is, at the time and place of patient care. This contrasts with the historical pattern in which testing was wholly or mostly confined to the medical laboratory, which entailed sending off specimens away from the point of care and then waiting hours or days to learn the results, during which time care must continue without the desired information.
Surface-enhanced laser desorption/ionization (SELDI) is a soft ionization method in mass spectrometry (MS) used for the analysis of protein mixtures. It is a variation of matrix-assisted laser desorption/ionization (MALDI). In MALDI, the sample is mixed with a matrix material and applied to a metal plate before irradiation by a laser, whereas in SELDI, proteins of interest in a sample become bound to a surface before MS analysis. The sample surface is a key component in the purification, desorption, and ionization of the sample. SELDI is typically used with time-of-flight (TOF) mass spectrometers and is used to detect proteins in tissue samples, blood, urine, or other clinical samples, however, SELDI technology can potentially be used in any application by simply modifying the sample surface.

A lateral flow test (LFT), is an assay also known as a lateral flow device (LFD), lateral flow immunochromatographic assay, or rapid test. It is a simple device intended to detect the presence of a target substance in a liquid sample without the need for specialized and costly equipment. LFTs are widely used in medical diagnostics in the home, at the point of care, and in the laboratory. For instance, the home pregnancy test is an LFT that detects a specific hormone. These tests are simple and economical and generally show results in around five to thirty minutes. Many lab-based applications increase the sensitivity of simple LFTs by employing additional dedicated equipment. Because the target substance is often a biological antigen, many lateral flow tests are rapid antigen tests (RAT or ART).
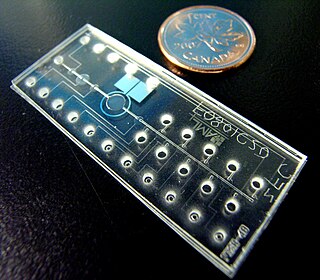
Bio-MEMS is an abbreviation for biomedical microelectromechanical systems. Bio-MEMS have considerable overlap, and is sometimes considered synonymous, with lab-on-a-chip (LOC) and micro total analysis systems (μTAS). Bio-MEMS is typically more focused on mechanical parts and microfabrication technologies made suitable for biological applications. On the other hand, lab-on-a-chip is concerned with miniaturization and integration of laboratory processes and experiments into single chips. In this definition, lab-on-a-chip devices do not strictly have biological applications, although most do or are amenable to be adapted for biological purposes. Similarly, micro total analysis systems may not have biological applications in mind, and are usually dedicated to chemical analysis. A broad definition for bio-MEMS can be used to refer to the science and technology of operating at the microscale for biological and biomedical applications, which may or may not include any electronic or mechanical functions. The interdisciplinary nature of bio-MEMS combines material sciences, clinical sciences, medicine, surgery, electrical engineering, mechanical engineering, optical engineering, chemical engineering, and biomedical engineering. Some of its major applications include genomics, proteomics, molecular diagnostics, point-of-care diagnostics, tissue engineering, single cell analysis and implantable microdevices.
Magnetic immunoassay (MIA) is a type of diagnostic immunoassay using magnetic beads as labels in lieu of conventional enzymes (ELISA), radioisotopes (RIA) or fluorescent moieties to detect a specified analyte. MIA involves the specific binding of an antibody to its antigen, where a magnetic label is conjugated to one element of the pair. The presence of magnetic beads is then detected by a magnetic reader (magnetometer) which measures the magnetic field change induced by the beads. The signal measured by the magnetometer is proportional to the analyte concentration in the initial sample.

The centrifugal micro-fluidic biochip or centrifugal micro-fluidic biodisk is a type of lab-on-a-chip technology, also known as lab-on-a-disc, that can be used to integrate processes such as separating, mixing, reaction and detecting molecules of nano-size in a single piece of platform, including a compact disk or DVD. This type of micro-fluidic biochip is based upon the principle of microfluidics; to take advantage of noninertial pumping for lab-on-a-chip devices using noninertial valves and switches under centrifugal force and Coriolis effect to distribute fluids about the disks in a highly parallel order.
Luminex Corporation | A DiaSorin Company is a biotechnology company which develops, manufactures and markets proprietary biological testing technologies with applications in life-sciences.
Flow-through tests or immunoconcentration assays are a type of diagnostic assay that allows users to test for the presence of a biomarker, usually a specific antibody, in a sample such as blood. They are a type of point of care test, a test designed to be used by a healthcare provider at patient contact. Point of care tests often allow for rapid detection of a specific biomarker without specialized lab equipment and training; this aids in diagnosis and allows therapeutic action to be initiated more quickly. Flow-through tests began development in the early 1980s and were the first type of immunostrip to be developed, although lateral flow tests have subsequently become the dominant immunostrip point of care device.
Ayoxxa Biosystems is a biotechnology company founded in 2010 in Singapore, and headquartered in Germany.

A compact disk/digital versatile disk (CD/DVD) based immunoassay is a method for determining the concentration of a compound in research and diagnostic laboratories by performing the test on an adapted CD/DVD surface using an adapted optical disc drive; these methods have been discussed and prototyped in research labs since 1991.
Paper-based microfluidics are microfluidic devices that consist of a series of hydrophilic cellulose or nitrocellulose fibers that transport fluid from an inlet through the porous medium to a desired outlet or region of the device, by means of capillary action. This technology builds on the conventional lateral flow test which is capable of detecting many infectious agents and chemical contaminants. The main advantage of this is that it is largely a passively controlled device unlike more complex microfluidic devices. Development of paper-based microfluidic devices began in the early 21st century to meet a need for inexpensive and portable medical diagnostic systems.
Aaron R. Wheeler is a Canadian chemist who is a professor of chemistry and biomedical engineering at the University of Toronto since 2005 with cross-appointment at Institute of Biomedical Engineering and Terrence Donnelly Centre for Cellular and Biomolecular Research. His academic laboratory is located at Lash Miller Chemical Laboratories and Terrence Donnelly Centre for Cellular and Biomolecular Research at the University of Toronto. In 2005, Wheeler was appointed as assistant professor and Tier II Canada Research Chair then promoted to associate professor in 2010, full professor in 2013, and in 2018 he became the Tier I Canada Research Chair in Microfluidic Bioanalysis.








