Related Research Articles

Muscular dystrophies (MD) are a genetically and clinically heterogeneous group of rare neuromuscular diseases that cause progressive weakness and breakdown of skeletal muscles over time. The disorders differ as to which muscles are primarily affected, the degree of weakness, how fast they worsen, and when symptoms begin. Some types are also associated with problems in other organs.
The National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) is one of the institutes and centers that make up the National Institutes of Health, an agency of the United States Department of Health and Human Services (HHS).
Muscular Dystrophy Association (MDA) is an American nonprofit organization dedicated to supporting people living with muscular dystrophy, ALS, and related neuromuscular diseases. Founded in 1950 by Paul Cohen, who lived with muscular dystrophy, MDA accelerates research, advances care, and works to empower families to live longer and more independent lives. Renowned for The MDA Labor Day Telethon, the annual telecast aired live from 1966 to 2010 and was hosted by Jerry Lewis, who also served as MDA's national chairman.
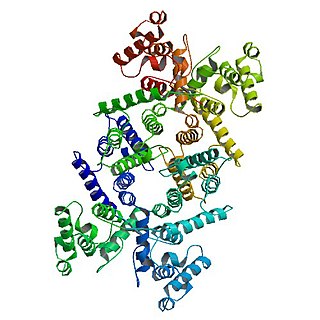
Dystrophin is a rod-shaped cytoplasmic protein, and a vital part of a protein complex that connects the cytoskeleton of a muscle fiber to the surrounding extracellular matrix through the cell membrane. This complex is variously known as the costamere or the dystrophin-associated protein complex (DAPC). Many muscle proteins, such as α-dystrobrevin, syncoilin, synemin, sarcoglycan, dystroglycan, and sarcospan, colocalize with dystrophin at the costamere. It has a molecular weight of 427 kDa

Duchenne muscular dystrophy (DMD) is a severe type of muscular dystrophy that primarily affects boys. Muscle weakness usually begins around the age of four, and worsens quickly. Muscle loss typically occurs first in the thighs and pelvis followed by the arms. This can result in trouble standing up. Most are unable to walk by the age of 12. Affected muscles may look larger due to increased fat content. Scoliosis is also common. Some may have intellectual disability. Females with a single copy of the defective gene may show mild symptoms.

Becker muscular dystrophy is an X-linked recessive inherited disorder characterized by slowly progressing muscle weakness of the legs and pelvis. It is a type of dystrophinopathy. This is caused by mutations in the dystrophin gene, which encodes the protein dystrophin. Becker muscular dystrophy is related to Duchenne muscular dystrophy in that both result from a mutation in the dystrophin gene, but has a milder course.

The Social Security Amendments of 1965, Pub. L.Tooltip Public Law 89–97, 79 Stat. 286, enacted July 30, 1965, was legislation in the United States whose most important provisions resulted in creation of two programs: Medicare and Medicaid. The legislation initially provided federal health insurance for the elderly and for financially challenged families.

Silvio Ottavio Conte was an American lawyer and politician. He was a Republican member of the United States House of Representatives for 16 terms, representing the 1st Congressional District of Massachusetts from January 3, 1959, until his death in Bethesda, Maryland in 1991. He strongly supported legislation to protect the environment, as well as federal funding of medical and scientific research.

The Public Health Service Act is a United States federal law enacted in 1944. The full act is codified in Title 42 of the United States Code, Chapter 6A . This Act provided a legislative basis for the provision of public health services in the United States.
MDCC may refer to:
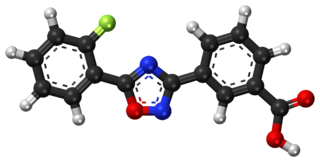
Ataluren, sold under the brand name Translarna, is a medication for the treatment of Duchenne muscular dystrophy. It was designed by PTC Therapeutics.
The Affordable Health Care for America Act was a bill that was crafted by the United States House of Representatives of the 111th United States Congress on October 29, 2009. The bill was sponsored by Representative Charles Rangel. At the encouragement of the Obama administration, the 111th Congress devoted much of its time to enacting reform of the United States' health care system. Known as the "House bill,” HR 3962 was the House of Representatives' chief legislative proposal during the health reform debate.
The Stupak–Pitts Amendment was a proposed amendment to the Affordable Health Care for America Act of 2010 (AHCAA). It was submitted by Representatives Bart Stupak and Joseph R. Pitts. Its stated purpose was to prohibit the use of federal funds "to pay for any abortion or to cover any part of the costs of any health plan that includes coverage of abortion" except in cases of rape, incest or danger to the life of the mother. It was adopted by the House but not included in the Senate's version, the Patient Protection and Affordable Care Act (PPACA). Representatives who support abortion rights said they would oppose AHCAA with the Stupak-Pitts language, and proposed to adopt PPACA. Stupak and several supporters said they would oppose PPACA without the amendment, but withdrew their opposition after President Obama promised an executive order to bar such funding. Anti-abortion groups criticized this action, saying that the executive order would not be effective.

The Health Care and Education Reconciliation Act of 2010 is a law that was enacted by the 111th United States Congress, by means of the reconciliation process, in order to amend the Affordable Care Act (ACA). The law includes the Student Aid and Fiscal Responsibility Act, which was attached as a rider.
The National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) is part of the United States National Institutes of Health, which in turn is part of the Department of Health and Human Services. NIDDK is approximately the fifth-largest of the 27 NIH institutes. The institute's mission is to support research, training, and communication with the public in the topic areas of "diabetes and other endocrine and metabolic diseases; digestive diseases, nutritional disorders, and obesity; and kidney, urologic, and hematologic diseases". As of 2021, the Director of the institute is Griffin P. Rodgers, who assumed the position on an acting basis in 2006 and on a permanent basis in 2007.

AIDS amendments of 1988, better known as the Health Omnibus Programs Extension (HOPE) Act of 1988, is a United States statute amending the Public Health Service Act. The Acquired Immune Deficiency Syndrome amendments were compiled as Title II - Programs with Respect to Acquired Immune Deficiency Syndrome within the HOPE Act of 1988. The Title II Act appropriated federal funding for Acquired Immune Deficiency Syndrome (AIDS) education, prevention, research, and testing. The U.S. legislative title provisioned the establishment of the presidentially appointed National Commission on AIDS. The S. 2889 legislation was passed by the 100th U.S. Congressional session and signed by President Ronald Reagan on November 4, 1988.

Marathon Pharmaceuticals LLC was a privately held biopharmaceuticals company focused on drugs for people with rare diseases. The Illinois-based company developed and manufactured therapeutics and brought them to market. It employed 100 people in four global locations. In 2017, PTC Therapeutics acquired rights to Marathon Pharmaceuticals' drug Emflaza (deflazacort) for $140 million after criticism about their plan to sell the drug at a list price of $89,000 per year to sufferers despite the fact that the same drug was available in Canada and the UK for around $1,000 per year.

The Newborn Screening Saves Lives Reauthorization Act of 2014 is a bill that would amend the Public Health Service Act to reauthorize grant programs and other initiatives to promote expanded screening of newborns and children for heritable disorders.

The Paul D. Wellstone Muscular Dystrophy Community Assistance, Research and Education Amendments of 2013 is a United States public law that amends the Public Health Service Act to revise the muscular dystrophy research program of the National Institutes of Health (NIH).
Toshifumi (Toshi) Yokota is a medical scientist and professor of medical genetics at the University of Alberta, where he also holds the titles of the Friends of Garrett Cumming Research & Muscular Dystrophy Canada Endowed Research Chair and the Henri M. Toupin Chair in Neurological Science. He is best known for his studies of antisense oligonucleotide-based therapeutics for muscular dystrophy that led to the development of an FDA-approved drug viltolarsen. His research interests include precision medicine for muscular dystrophy and genetic diseases. He has co-edited two books both published in the Methods in Molecular Biology series from Humana Press, Springer-Nature, and has published more than 100 refereed papers and patents. He is a member of the editorial boards for the International Journal of Molecular Sciences, Genes, Frontiers in Genome Editing, Frontiers in Physiology, and Nucleic Acid Therapeutics, a member of the Medical and Scientific Advisory Committee of Muscular Dystrophy Canada, and a co-founder of the Canadian Neuromuscular Network (CAN-NMD).
References
- ↑ H.R. 717--107th Congress (2001): MD-CARE Act, GovTrack.us (database of federal legislation), (accessed July 29, 2007)
- 1 2 Public Law 107-84, PDF as retrieved from NIH website
- ↑ MDCC website, NIH, Last updated May 18, 2007
- 1 2 "H.R. 594 - Summary". United States Congress. Retrieved 30 July 2014.
- ↑ "H.R. 594 - All Actions". United States Congress. Retrieved 31 July 2014.