Glycosaminoglycans (GAGs) or mucopolysaccharides are long linear polysaccharides consisting of repeating disaccharide units. The repeating two-sugar unit consists of a uronic sugar and an amino sugar, with the exception of keratan, where in the place of the uronic sugar it has galactose. Because GAGs are highly polar and attract water, they are used in the body as a lubricant or shock absorber.

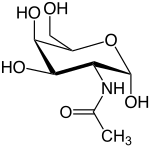
N-Acetylglucosamine (GlcNAc) is an amide derivative of the monosaccharide glucose. It is a secondary amide between glucosamine and acetic acid. It is significant in several biological systems.

Heparan sulfate (HS) is a linear polysaccharide found in all animal tissues. It occurs as a proteoglycan in which two or three HS chains are attached in close proximity to cell surface or extracellular matrix proteins. It is in this form that HS binds to a variety of protein ligands, including Wnt, and regulates a wide range of biological activities, including developmental processes, angiogenesis, blood coagulation, abolishing detachment activity by GrB, and tumour metastasis. HS has also been shown to serve as cellular receptor for a number of viruses, including the respiratory syncytial virus. A recent study reports that cellular heparan sulfate has a role in SARS-CoV-2 Infection, particularly when the virus attaches with ACE2.

Hexosaminidase is an enzyme involved in the hydrolysis of terminal N-acetyl-D-hexosamine residues in N-acetyl-β-D-hexosaminides.

The enzyme UDP-glucose 4-epimerase, also known as UDP-galactose 4-epimerase or GALE, is a homodimeric epimerase found in bacterial, fungal, plant, and mammalian cells. This enzyme performs the final step in the Leloir pathway of galactose metabolism, catalyzing the reversible conversion of UDP-galactose to UDP-glucose. GALE tightly binds nicotinamide adenine dinucleotide (NAD+), a co-factor required for catalytic activity.
In enzymology, a globotriaosylceramide 3-beta-N-acetylgalactosaminyltransferase is an enzyme that catalyzes the chemical reaction
In enzymology, a N-acetylgalactosamine kinase is an enzyme that catalyzes the chemical reaction

Polypeptide N-acetylgalactosaminyltransferase 3 is an enzyme that in humans is encoded by the GALNT3 gene.

Polypeptide N-acetylgalactosaminyltransferase 1 is an enzyme that in humans is encoded by the GALNT1 gene.

Polypeptide N-acetylgalactosaminyltransferase 2 is an enzyme that in humans is encoded by the GALNT2 gene.

UDP-GalNAc:beta-1,3-N-acetylgalactosaminyltransferase 1 is an enzyme that in humans is encoded by the B3GALNT1 gene.

Polypeptide N-acetylgalactosaminyltransferase 6 is an enzyme that in humans is encoded by the GALNT6 gene.

Polypeptide N-acetylgalactosaminyltransferase 13 is an enzyme that in humans is encoded by the GALNT13 gene.

Alpha-N-acetylgalactosaminide alpha-2,6-sialyltransferase 1 is an enzyme that in humans is encoded by the ST6GALNAC1 gene. This enzyme adds a N-Acetylneuraminic acid to an O-linked N-Acetylgalactosamine (GalNAc) on a peptide/proteins with an α2-6 linkage to produce the sialyl-Tn antigen. It has been shown that the enzyme prefers Thr over Ser containing GalNAc residues.
Histo-blood group ABO system transferase is an enzyme with glycosyltransferase activity, which is encoded by the ABO gene in humans. It is ubiquitously expressed in many tissues and cell types. ABO determines the ABO blood group of an individual by modifying the oligosaccharides on cell surface glycoproteins. Variations in the sequence of the protein between individuals determine the type of modification and the blood group. The ABO gene also contains one of 27 SNPs associated with increased risk of coronary artery disease.
Core 1 synthase, glycoprotein-N-acetylgalactosamine 3-beta-galactosyltransferase, 1, also known as C1GALT1, is an enzyme which in humans is encoded by the C1GALT1 gene.

Carbohydrate sulfotransferases are sulfotransferase enzymes that transfer sulfate to carbohydrate groups in glycoproteins and glycolipids. Carbohydrates are used by cells for a wide range of functions from structural purposes to extracellular communication. Carbohydrates are suitable for such a wide variety of functions due to the diversity in structure generated from monosaccharide composition, glycosidic linkage positions, chain branching, and covalent modification. Possible covalent modifications include acetylation, methylation, phosphorylation, and sulfation. Sulfation, performed by carbohydrate sulfotransferases, generates carbohydrate sulfate esters. These sulfate esters are only located extracellularly, whether through excretion into the extracellular matrix (ECM) or by presentation on the cell surface. As extracellular compounds, sulfated carbohydrates are mediators of intercellular communication, cellular adhesion, and ECM maintenance.
O-linked glycosylation is the attachment of a sugar molecule to the oxygen atom of serine (Ser) or threonine (Thr) residues in a protein. O-glycosylation is a post-translational modification that occurs after the protein has been synthesised. In eukaryotes, it occurs in the endoplasmic reticulum, Golgi apparatus and occasionally in the cytoplasm; in prokaryotes, it occurs in the cytoplasm. Several different sugars can be added to the serine or threonine, and they affect the protein in different ways by changing protein stability and regulating protein activity. O-glycans, which are the sugars added to the serine or threonine, have numerous functions throughout the body, including trafficking of cells in the immune system, allowing recognition of foreign material, controlling cell metabolism and providing cartilage and tendon flexibility. Because of the many functions they have, changes in O-glycosylation are important in many diseases including cancer, diabetes and Alzheimer's. O-glycosylation occurs in all domains of life, including eukaryotes, archaea and a number of pathogenic bacteria including Burkholderia cenocepacia, Neisseria gonorrhoeae and Acinetobacter baumannii.

O-GlcNAc is a reversible enzymatic post-translational modification that is found on serine and threonine residues of nucleocytoplasmic proteins. The modification is characterized by a β-glycosidic bond between the hydroxyl group of serine or threonine side chains and N-acetylglucosamine (GlcNAc). O-GlcNAc differs from other forms of protein glycosylation: (i) O-GlcNAc is not elongated or modified to form more complex glycan structures, (ii) O-GlcNAc is almost exclusively found on nuclear and cytoplasmic proteins rather than membrane proteins and secretory proteins, and (iii) O-GlcNAc is a highly dynamic modification that turns over more rapidly than the proteins which it modifies. O-GlcNAc is conserved across metazoans.

The bump-and-hole method is a tool in chemical genetics for studying a specific isoform in a protein family without perturbing the other members of the family. The unattainability of isoform-selective inhibition due to structural homology in protein families is a major challenge of chemical genetics. With the bump-and-hole approach, a protein–ligand interface is engineered to achieve selectivity through steric complementarity while maintaining biochemical competence and orthogonality to the wild type pair. Typically, a "bumped" ligand/inhibitor analog is designed to bind a corresponding "hole-modified" protein. Bumped ligands are commonly bulkier derivatives of a cofactor of the target protein. Hole-modified proteins are recombinantly expressed with an amino acid substitution from a larger to smaller residue, e.g. glycine or alanine, at the cofactor binding site. The designed ligand/inhibitor has specificity for the engineered protein due to steric complementarity, but not the native counterpart due to steric interference.