
Haematoxylin or hematoxylin, also called natural black 1 or C.I. 75290, is a compound extracted from heartwood of the logwood tree with a chemical formula of C
16H
14O
6. This naturally derived dye has been used as a histologic stain, as an ink and as a dye in the textile and leather industry. As a dye, haematoxylin has been called palo de Campeche, logwood extract, bluewood and blackwood. In histology, haematoxylin staining is commonly followed by counterstaining with eosin. When paired, this staining procedure is known as H&E staining and is one of the most commonly used combinations in histology. In addition to its use in the H&E stain, haematoxylin is also a component of the Papanicolaou stain which is widely used in the study of cytology specimens.

Potassium ferricyanide is the chemical compound with the formula K3[Fe(CN)6]. This bright red salt contains the octahedrally coordinated [Fe(CN)6]3− ion. It is soluble in water and its solution shows some green-yellow fluorescence. It was discovered in 1822 by Leopold Gmelin.

Staining is a technique used to enhance contrast in samples, generally at the microscopic level. Stains and dyes are frequently used in histology, in cytology, and in the medical fields of histopathology, hematology, and cytopathology that focus on the study and diagnoses of diseases at the microscopic level. Stains may be used to define biological tissues, cell populations, or organelles within individual cells.
Iron(III) chloride describes the inorganic compounds with the formula FeCl3(H2O)x. Also called ferric chloride, these compounds are some of the most important and commonplace compounds of iron. They are available both in anhydrous and in hydrated forms which are both hygroscopic. They feature iron in its +3 oxidation state. The anhydrous derivative is a Lewis acid, while all forms are mild oxidizing agents. It is used as a water cleaner and as an etchant for metals.

Chromium(III) chloride (also called chromic chloride) is an inorganic chemical compound with the chemical formula CrCl3. It forms several hydrates with the formula CrCl3·nH2O, among which are hydrates where n can be 5 (chromium(III) chloride pentahydrate CrCl3·5H2O) or 6 (chromium(III) chloride hexahydrate CrCl3·6H2O). The anhydrous compound with the formula CrCl3 are violet crystals, while the most common form of the chromium(III) chloride are the dark green crystals of hexahydrate, CrCl3·6H2O. Chromium chlorides find use as catalysts and as precursors to dyes for wool.

Nile blue is a stain used in biology and histology. It may be used with live or fixed cells, and imparts a blue colour to cell nuclei.

An acid dye is a dye that is typically applied to a textile at low pH. They are mainly used to dye wool, not cotton fabrics. Some acid dyes are used as food colorants, and some can also be used to stain organelles in the medical field.

Iron(II) acetate is a coordination complex with formula Fe(CH3COO)2. It is a white solid, although impure samples can be slightly colored. A light green tetrahydrate is also known, which is highly soluble in water.

Hematoxylin and eosin stain is one of the principal tissue stains used in histology. It is the most widely used stain in medical diagnosis and is often the gold standard. For example, when a pathologist looks at a biopsy of a suspected cancer, the histological section is likely to be stained with H&E.

Phosphotungstic acid (PTA) or tungstophosphoric acid (TPA), is a heteropoly acid with the chemical formula H3PW12O40]. It forms hydrates H3[PW12O40]·nH2O. It is normally isolated as the n = 24 hydrate but can be desiccated to the hexahydrate (n = 6). EPTA is the name of ethanolic phosphotungstic acid, its alcohol solution used in biology. It has the appearance of small, colorless-grayish or slightly yellow-green crystals, with melting point 89 °C (24 H2O hydrate). It is odorless and soluble in water (200 g/100 ml). It is not especially toxic, but is a mild acidic irritant. The compound is known by a variety of names and acronyms (see 'other names' section of infobox).
Sudan stains and Sudan dyes are synthetic organic compounds that are used as dyes for various plastics and are also used to stain sudanophilic biological samples, usually lipids. Sudan II, Sudan III, Sudan IV, Oil Red O, and Sudan Black B are important members of this class of compounds.

In organic chemistry, nitroso refers to a functional group in which the nitric oxide group is attached to an organic moiety. As such, various nitroso groups can be categorized as C-nitroso compounds, S-nitroso compounds, N-nitroso compounds, and O-nitroso compounds.

Metal nitrosyl complexes are complexes that contain nitric oxide, NO, bonded to a transition metal. Many kinds of nitrosyl complexes are known, which vary both in structure and coligand.

Toluidine blue, also known as TBO or tolonium chloride (INN) is a blue cationic (basic) dye used in histology and sometimes clinically.

2-Naphthol, or β-naphthol, is a fluorescent colorless (or occasionally yellow) crystalline solid with the formula C10H7OH. It is an isomer of 1-naphthol, differing by the location of the hydroxyl group on the naphthalene ring. The naphthols are naphthalene homologues of phenol, but more reactive. Both isomers are soluble in simple alcohols, ethers, and chloroform. 2-Naphthol is a widely used intermediate for the production of dyes and other compounds.
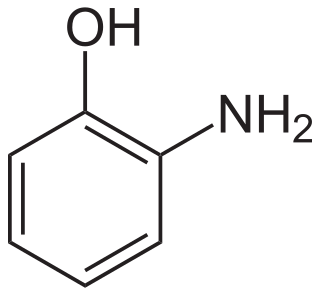
2-Aminophenol is an organic compound with the formula C6H7NO. Along with its isomer 4-aminophenol, it is an amphoteric molecule and a reducing agent. It is a useful reagent for the synthesis of dyes and heterocyclic compounds. Reflecting its slight hydrophilic character, white powder is moderately soluble in alcohols and can be recrystallized from hot water.
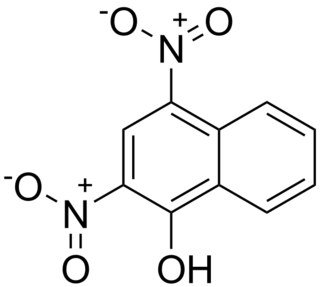
Martius yellow is an organic compound that once was used to protect wool from moths. It is prepared by nitration of naphthol.

Alcian blue is any member of a family of polyvalent basic dyes, of which the Alcian blue 8G has been historically the most common and the most reliable member. It is used to stain acidic polysaccharides such as glycosaminoglycans in cartilages and other body structures, some types of mucopolysaccharides, sialylated glycocalyx of cells etc. For many of these targets it is one of the most widely used cationic dyes for both light and electron microscopy. Use of alcian blue has historically been a popular staining method in histology especially for light microscopy in paraffin embedded sections and in semithin resin sections. The tissue parts that specifically stain by this dye become blue to bluish-green after staining and are called "Alcianophilic". Alcian blue staining can be combined with H&E staining, PAS staining and van Gieson staining methods. Alcian blue can be used to quantitate acidic glycans both in microspectrophotometric quantitation in solution or for staining glycoproteins in polyacrylamide gels or on western blots. Biochemists had used it to assay acid polysaccharides in urine since the 1960s for diagnosis of diseases like mucopolysaccharidosis but from 1970's, partly due to lack of availability of Alcian and partly due to length and tediousness of the procedure, alternative methods had to be developed e.g. Dimethyl methylene blue method.
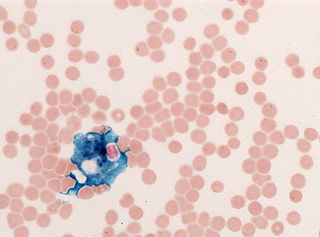
In histology, histopathology, and clinical pathology, Perls Prussian blue is a commonly used method to detect the presence of iron in tissue or cell samples. Perls Prussian Blue derives its name from the German pathologist Max Perls (1843–1881), who described the technique in 1867. The method does not involve the application of a dye, but rather causes the pigment Prussian blue to form directly within the tissue. The method stains mostly iron in the ferric state which includes ferritin and hemosiderin, rather than iron in the ferrous state.

1-Nitroso-2-naphthol is an organic compound with the formula C10H6(NO)OH. It is one of several possible nitrosonaphthols, and the most studied for applications as an indicator and a dye.


















