
Acetylcholine (ACh) is an organic compound that functions in the brain and body of many types of animals as a neurotransmitter. Its name is derived from its chemical structure: it is an ester of acetic acid and choline. Parts in the body that use or are affected by acetylcholine are referred to as cholinergic.

Soman is an extremely toxic chemical substance. It is a nerve agent, interfering with normal functioning of the mammalian nervous system by inhibiting the enzyme cholinesterase. It is an inhibitor of both acetylcholinesterase and butyrylcholinesterase. As a chemical weapon, it is classified as a weapon of mass destruction by the United Nations according to UN Resolution 687. Its production is strictly controlled, and stockpiling is outlawed by the Chemical Weapons Convention of 1993 where it is classified as a Schedule 1 substance. Soman was the third of the so-called G-series nerve agents to be discovered along with GA (tabun), GB (sarin), and GF (cyclosarin).
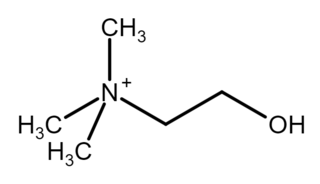
Choline is a cation with the chemical formula [(CH3)3NCH2CH2OH]+. Choline forms various salts, for example choline chloride and choline bitartrate.

The enzyme cholinesterase (EC 3.1.1.8, choline esterase; systematic name acylcholine acylhydrolase) catalyses the hydrolysis of choline-based esters:

Choline acetyltransferase is a transferase enzyme responsible for the synthesis of the neurotransmitter acetylcholine. ChAT catalyzes the transfer of an acetyl group from the coenzyme acetyl-CoA to choline, yielding acetylcholine (ACh). ChAT is found in high concentration in cholinergic neurons, both in the central nervous system (CNS) and peripheral nervous system (PNS). As with most nerve terminal proteins, ChAT is produced in the body of the neuron and is transported to the nerve terminal, where its concentration is highest. Presence of ChAT in a nerve cell classifies this cell as a "cholinergic" neuron. In humans, the choline acetyltransferase enzyme is encoded by the CHAT gene.
A parasympathomimetic drug, sometimes called a cholinomimetic drug or cholinergic receptor stimulating agent, is a substance that stimulates the parasympathetic nervous system (PSNS). These chemicals are also called cholinergic drugs because acetylcholine (ACh) is the neurotransmitter used by the PSNS. Chemicals in this family can act either directly by stimulating the nicotinic or muscarinic receptors, or indirectly by inhibiting cholinesterase, promoting acetylcholine release, or other mechanisms. Common uses of parasympathomimetics include glaucoma, Sjögren syndrome and underactive bladder.

Coluracetam is a purported nootropic agent of the racetam family. It is contains a chemical group that is a bioisostere of the 9-amino-tetrahydroacridine family. It was initially developed and tested by the Mitsubishi Tanabe Pharma Corporation for Alzheimer's disease. After the drug failed to reach endpoints in its clinical trials it was in-licensed by BrainCells Inc for investigations into major depressive disorder (MDD), which was preceded by being awarded a "Qualifying Therapeutic Discovery Program Grant" by the state of California. Findings from phase IIa clinical trials have suggested that it would be a potential medication for comorbid MDD with generalized anxiety disorder (GAD). BrainCells Inc is currently out-licensing the drug for this purpose. It may also have potential use in prevention and treatment of ischemic retinopathy and retinal and optic nerve injury.

Huperzine A is a naturally occurring sesquiterpene alkaloid compound found in the firmoss Huperzia serrata and in varying quantities in other food Huperzia species, including H. elmeri, H. carinat, and H. aqualupian. Huperzine A has been investigated as a treatment for neurological conditions such as Alzheimer's disease, but a meta-analysis of those studies concluded that they were of poor methodological quality and the findings should be interpreted with caution. Huperzine A inhibits the breakdown of the neurotransmitter acetylcholine by the enzyme acetylcholinesterase. It is commonly available over the counter as a nutrient supplement, and is marketed as a cognitive enhancer for improving memory and concentration.

Aralkylamine N-acetyltransferase (AANAT), also known as arylalkylamine N-acetyltransferase or serotonin N-acetyltransferase (SNAT), is an enzyme that is involved in the day/night rhythmic production of melatonin, by modification of serotonin. It is in humans encoded by the ~2.5 kb AANAT gene containing four exons, located on chromosome 17q25. The gene is translated into a 23 kDa large enzyme. It is well conserved through evolution and the human form of the protein is 80 percent identical to sheep and rat AANAT. It is an acetyl-CoA-dependent enzyme of the GCN5-related family of N-acetyltransferases (GNATs). It may contribute to multifactorial genetic diseases such as altered behavior in sleep/wake cycle and research is on-going with the aim of developing drugs that regulate AANAT function.

The Vesicular acetylcholine transporter (VAChT) is a neurotransmitter transporter which is responsible for loading acetylcholine (ACh) into secretory organelles in neurons making acetylcholine available for secretion. It is encoded by Solute carrier family 18, member 3 (SLC18A3) gene, located within the first intron of the choline acetyltransferase gene. VAChT is able to transport ACh into vesicles by relying on an exchange between protons (H+) that were previously pumped into the vesicle diffusing out, thus acting as an antiporter. ACh molecules are then carried into the vesicle by the action of exiting protons. Acetylcholine transport utilizes a proton gradient established by a vacuolar ATPase.
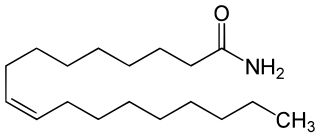
Oleamide is an organic compound with the formula CH3(CH2)7CH=CH(CH2)7CONH2. It is the amide derived from the fatty acid oleic acid. It is a colorless waxy solid and occurs in nature. Sometimes labeled as a fatty acid primary amide (FAPA), it is biosynthesized from N-oleoylglycine.

Acetylcholinesterase (HGNC symbol ACHE; EC 3.1.1.7; systematic name acetylcholine acetylhydrolase), also known as AChE, AChase or acetylhydrolase, is the primary cholinesterase in the body. It is an enzyme that catalyzes the breakdown of acetylcholine and some other choline esters that function as neurotransmitters:

Choline transporter-like protein 1 is a protein that in humans is encoded by the SLC44A1 gene.

A reuptake inhibitor (RI) is a type of drug known as a reuptake modulator that inhibits the plasmalemmal transporter-mediated reuptake of a neurotransmitter from the synapse into the pre-synaptic neuron. This leads to an increase in extracellular concentrations of the neurotransmitter and an increase in neurotransmission. Various drugs exert their psychological and physiological effects through reuptake inhibition, including many antidepressants and psychostimulants.

Tricyanoaminopropene is a nootropic drug which mimics the function of nerve growth factor and increases the growth of nerves and tissue regeneration both in isolated tissues and in vivo. It stimulates the action of the enzyme choline acetyltransferase, resulting in increased acetylcholine production. This then results in increased synthesis of RNA in many different tissues in the body. However it also suppresses the production of thyroxine, causing temporary hypothyroidism which returns to normal once the drug is discontinued.

Bifemelane (INN) (Alnert, Celeport), or bifemelane hydrochloride (JAN), also known as 4-(O-benzylphenoxy)-N-methylbutylamine, is an antidepressant and cerebral activator that is widely used in the treatment of cerebral infarction patients with depressive symptoms in Japan, and in the treatment of senile dementia as well. It also appears to be useful in the treatment of glaucoma. Bifemelane acts as a monoamine oxidase inhibitor (MAOI) of both isoenzymes, with competitive (reversible) inhibition of MAO-A (Ki = 4.20 μM) (making it a reversible inhibitor of monoamine oxidase A (RIMA)) and non-competitive (irreversible) inhibition of MAO-B (Ki = 46.0 μM), and also acts (weakly) as a norepinephrine reuptake inhibitor. The drug has nootropic, neuroprotective, and antidepressant-like effects in animal models, and appears to enhance the cholinergic system in the brain.

A reuptake enhancer (RE), also sometimes referred to as a reuptake activator, is a type of reuptake modulator which enhances the plasmalemmal transporter-mediated reuptake of a neurotransmitter from the synapse into the pre-synaptic neuron, leading to a decrease in the extracellular concentrations of the neurotransmitter and therefore a decrease in neurotransmission.

Tetraethyl pyrophosphate, abbreviated TEPP, is an organophosphate compound with the formula [(C2H5O)2P(O)]2O. It is the tetraethyl derivative of pyrophosphate (P2O74-). It is a colorless oil that solidifies near room temperature. It is used as an insecticide. The compound hydrolyzes rapidly.

A cholinergic neuron is a nerve cell which mainly uses the neurotransmitter acetylcholine (ACh) to send its messages. Many neurological systems are cholinergic. Cholinergic neurons provide the primary source of acetylcholine to the cerebral cortex, and promote cortical activation during both wakefulness and rapid eye movement sleep. The cholinergic system of neurons has been a main focus of research in aging and neural degradation, specifically as it relates to Alzheimer's disease. The dysfunction and loss of basal forebrain cholinergic neurons and their cortical projections are among the earliest pathological events in Alzheimer's disease.

Methylfluorophosphonylcholine (MFPCh) is an extremely toxic chemical compound related to the G-series nerve agents. It is an extremely potent acetylcholinesterase inhibitor which is around 100 times more potent than sarin at inhibiting acetylcholinesterase in vitro, and around 10 times more potent in vivo, depending on route of administration and animal species tested. MFPCh is resistant to oxime reactivators, meaning the acetylcholinesterase inhibited by MFPCh can't be reactivated by cholinesterase reactivators. MFPCh also acts directly on the acetylcholine receptors. However, despite its high toxicity, methylfluorophosphonylcholine is a relatively unstable compound and degrades rapidly in storage, so it was not deemed suitable to be weaponised for military use.


















