Related Research Articles

Caenorhabditis elegans is a free-living transparent nematode about 1 mm in length that lives in temperate soil environments. It is the type species of its genus. The name is a blend of the Greek caeno- (recent), rhabditis (rod-like) and Latin elegans (elegant). In 1900, Maupas initially named it Rhabditides elegans. Osche placed it in the subgenus Caenorhabditis in 1952, and in 1955, Dougherty raised Caenorhabditis to the status of genus.

Rhodopsin, also known as visual purple, is a protein encoded by the RHO gene and a G-protein-coupled receptor (GPCR). It is the opsin of the rod cells in the retina and a light-sensitive receptor protein that triggers visual phototransduction in rods. Rhodopsin mediates dim light vision and thus is extremely sensitive to light. When rhodopsin is exposed to light, it immediately photobleaches. In humans, it is regenerated fully in about 30 minutes, after which the rods are more sensitive. Defects in the rhodopsin gene cause eye diseases such as retinitis pigmentosa and congenital stationary night blindness.
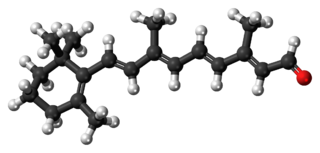
Retinal is a polyene chromophore. Retinal, bound to proteins called opsins, is the chemical basis of visual phototransduction, the light-detection stage of visual perception (vision).

Melanopsin is a type of photopigment belonging to a larger family of light-sensitive retinal proteins called opsins and encoded by the gene Opn4. In the mammalian retina, there are two additional categories of opsins, both involved in the formation of visual images: rhodopsin and photopsin in the rod and cone photoreceptor cells, respectively.

Proteorhodopsin is a family of transmembrane proteins that use retinal as a chromophore for light-mediated functionality, in this case, a proton pump. pRhodopsin is found in marine planktonic bacteria, archaea and eukaryotes (protae), but was first discovered in bacteria.
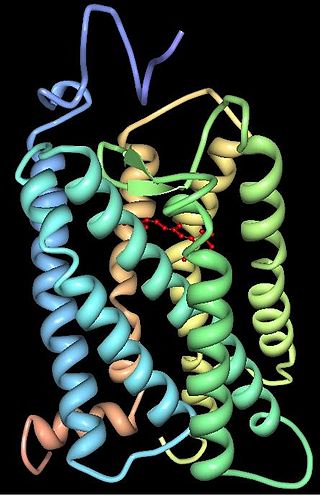
Animal opsins are G-protein-coupled receptors and a group of proteins made light-sensitive via a chromophore, typically retinal. When bound to retinal, opsins become retinylidene proteins, but are usually still called opsins regardless. Most prominently, they are found in photoreceptor cells of the retina. Five classical groups of opsins are involved in vision, mediating the conversion of a photon of light into an electrochemical signal, the first step in the visual transduction cascade. Another opsin found in the mammalian retina, melanopsin, is involved in circadian rhythms and pupillary reflex but not in vision. Humans have in total nine opsins. Beside vision and light perception, opsins may also sense temperature, sound, or chemicals.
Channelrhodopsins are a subfamily of retinylidene proteins (rhodopsins) that function as light-gated ion channels. They serve as sensory photoreceptors in unicellular green algae, controlling phototaxis: movement in response to light. Expressed in cells of other organisms, they enable light to control electrical excitability, intracellular acidity, calcium influx, and other cellular processes. Channelrhodopsin-1 (ChR1) and Channelrhodopsin-2 (ChR2) from the model organism Chlamydomonas reinhardtii are the first discovered channelrhodopsins. Variants that are sensitive to different colors of light or selective for specific ions have been cloned from other species of algae and protists.
Rhodopsin kinase is a serine/threonine-specific protein kinase involved in phototransduction. This enzyme catalyses the following chemical reaction:
Retinylidene proteins, or rhodopsins in a broad sense, are proteins that use retinal as a chromophore for light reception. They are the molecular basis for a variety of light-sensing systems from phototaxis in flagellates to eyesight in animals. Retinylidene proteins include all forms of opsin and rhodopsin. While rhodopsin in the narrow sense refers to a dim-light visual pigment found in vertebrates, usually on rod cells, rhodopsin in the broad sense refers to any molecule consisting of an opsin and a retinal chromophore in the ground state. When activated by light, the chromophore is isomerized, at which point the molecule as a whole is no longer rhodopsin, but a related molecule such as metarhodopsin. However, it remains a retinylidene protein. The chromophore then separates from the opsin, at which point the bare opsin is a retinylidene protein. Thus, the molecule remains a retinylidene protein throughout the phototransduction cycle.
The visual cycle is a process in the retina that replenishes the molecule retinal for its use in vision. Retinal is the chromophore of most visual opsins, meaning it captures the photons to begin the phototransduction cascade. When the photon is absorbed, the 11-cis retinal photoisomerizes into all-trans retinal as it is ejected from the opsin protein. Each molecule of retinal must travel from the photoreceptor cell to the RPE and back in order to be refreshed and combined with another opsin. This closed enzymatic pathway of 11-cis retinal is sometimes called Wald's visual cycle after George Wald (1906–1997), who received the Nobel Prize in 1967 for his work towards its discovery.

Peropsin, a visual pigment-like receptor, is a protein that in humans is encoded by the RRH gene. It belongs like other animal opsins to the G protein-coupled receptors. Even so, the first peropsins were already discovered in mice and humans in 1997, not much is known about them.

Opsin-5, also known as G-protein coupled receptor 136 or neuropsin is a protein that in humans is encoded by the OPN5 gene. Opsin-5 is a member of the opsin subfamily of the G protein-coupled receptors. It is a photoreceptor protein sensitive to ultraviolet (UV) light. The OPN5 gene was discovered in mouse and human genomes and its mRNA expression was also found in neural tissues. Neuropsin is bistable at 0 °C and activates a UV-sensitive, heterotrimeric G protein Gi-mediated pathway in mammalian and avian tissues.

RPE-retinal G protein-coupled receptor also known as RGR-opsin is a protein that in humans is encoded by the RGR gene. RGR-opsin is a member of the rhodopsin-like receptor subfamily of GPCR. Like other opsins which bind retinaldehyde, it contains a conserved lysine residue in the seventh transmembrane domain. RGR-opsin comes in different isoforms produced by alternative splicing.

Retinal degeneration is a retinopathy which consists in the deterioration of the retina caused by the progressive death of its cells. There are several reasons for retinal degeneration, including artery or vein occlusion, diabetic retinopathy, R.L.F./R.O.P., or disease. These may present in many different ways such as impaired vision, night blindness, retinal detachment, light sensitivity, tunnel vision, and loss of peripheral vision to total loss of vision. Of the retinal degenerative diseases retinitis pigmentosa (RP) is a very important example.
lsy-6 microRNA belongs to the class of miRNAs; these function to regulate the expression levels of other genes by several mechanisms. lsy-6 is a short non-coding RNA molecule and the first miRNA identified as having a role in nervous system development. It regulates left-right neuronal asymmetry in the nematode worm Caenorhabditis elegans.
Microbial rhodopsins, also known as bacterial rhodopsins, are retinal-binding proteins that provide light-dependent ion transport and sensory functions in halophilic and other bacteria. They are integral membrane proteins with seven transmembrane helices, the last of which contains the attachment point for retinal. Most microbial rhodopsins pump inwards, however "mirror rhodopsins" which function outwards. have been discovered.

Archaerhodopsin proteins are a family of retinal-containing photoreceptors found in the archaea genera Halobacterium and Halorubrum. Like the homologous bacteriorhodopsin (bR) protein, archaerhodopsins harvest energy from sunlight to pump H+ ions out of the cell, establishing a proton motive force that is used for ATP synthesis. They have some structural similarities to the mammalian G protein-coupled receptor protein rhodopsin, but are not true homologs.

William Ronald Schafer is a neuroscientist and geneticist who has made important contributions to understanding the molecular and neural basis of behaviour. His work, principally in the nematode C. elegans, has used an interdisciplinary approach to investigate how small groups of neurons generate behavior, and he has pioneered methodological approaches, including optogenetic neuroimaging and automated behavioural phenotyping, that have been widely influential in the broader neuroscience field. He has made significant discoveries on the functional properties of ionotropic receptors in sensory transduction and on the roles of gap junctions and extrasynaptic modulation in neuronal microcircuits. More recently, he has applied theoretical ideas from network science and control theory to investigate the structure and function of simple neuronal connectomes, with the goal of understanding conserved computational principles in larger brains. He is an EMBO member, Welcome Investigator and Fellow of the Academy of Medical Sciences.
Paul W. Sternberg is an American biologist. He does research for WormBase on C. elegans, a model organism.

Vertebrate visual opsins are a subclass of ciliary opsins and mediate vision in vertebrates. They include the opsins in human rod and cone cells. They are often abbreviated to opsin, as they were the first opsins discovered and are still the most widely studied opsins.
References
- 1 2 3 4 5 6 Troemel ER, Chou JH, Dwyer ND, Colbert HA, Bargmann CI (October 1995). "Divergent seven transmembrane receptors are candidate chemosensory receptors in C. elegans". Cell. 83 (2): 207–218. doi: 10.1016/0092-8674(95)90162-0 . PMID 7585938.
- 1 2 Robertson HM, Thomas JH (January 2006). "The putative chemoreceptor families of C. elegans". WormBook: 1–12. doi:10.1895/wormbook.1.66.1. PMC 4781013 . PMID 18050473.
- ↑ Chen N, Pai S, Zhao Z, Mah A, Newbury R, Johnsen RC, et al. (January 2005). "Identification of a nematode chemosensory gene family". Proceedings of the National Academy of Sciences of the United States of America. 102 (1): 146–151. Bibcode:2005PNAS..102..146C. doi: 10.1073/pnas.0408307102 . PMC 539308 . PMID 15618405.
- ↑ Nordström KJ, Sällman Almén M, Edstam MM, Fredriksson R, Schiöth HB (September 2011). "Independent HHsearch, Needleman--Wunsch-based, and motif analyses reveal the overall hierarchy for most of the G protein-coupled receptor families". Molecular Biology and Evolution. 28 (9): 2471–2480. doi:10.1093/molbev/msr061. PMID 21402729.
- 1 2 Gühmann M, Porter ML, Bok MJ (August 2022). "The Gluopsins: Opsins without the Retinal Binding Lysine". Cells. 11 (15): 2441. doi: 10.3390/cells11152441 . PMC 9368030 . PMID 35954284.
- ↑ Bownds D (December 1967). "Site of attachment of retinal in rhodopsin". Nature. 216 (5121): 1178–1181. Bibcode:1967Natur.216.1178B. doi:10.1038/2161178a0. PMID 4294735. S2CID 1657759.
- ↑ Collins FD (March 1953). "Rhodopsin and indicator yellow". Nature. 171 (4350): 469–471. Bibcode:1953Natur.171..469C. doi:10.1038/171469a0. PMID 13046517. S2CID 4152360.
- ↑ Pitt GA, Collins FD, Morton RA, Stok P (January 1955). "Studies on rhodopsin. VIII. Retinylidenemethylamine, an indicator yellow analogue". The Biochemical Journal. 59 (1): 122–128. doi:10.1042/bj0590122. PMC 1216098 . PMID 14351151.
- ↑ Leung NY, Thakur DP, Gurav AS, Kim SH, Di Pizio A, Niv MY, Montell C (April 2020). "Functions of Opsins in Drosophila Taste". Current Biology. 30 (8): 1367–1379.e6. Bibcode:2020CBio...30E1367L. doi:10.1016/j.cub.2020.01.068. PMC 7252503 . PMID 32243853.
- ↑ Kumbalasiri T, Rollag MD, Isoldi MC, Castrucci AM, Provencio I (March 2007). "Melanopsin triggers the release of internal calcium stores in response to light". Photochemistry and Photobiology. 83 (2): 273–279. doi:10.1562/2006-07-11-RA-964. PMID 16961436. S2CID 23060331.