
Kurt Wüthrich is a Swiss chemist/biophysicist and Nobel Chemistry laureate, known for developing nuclear magnetic resonance (NMR) methods for studying biological macromolecules.
Vratislavice nad Nisou is a self-governing borough of the city of Liberec in the Czech Republic. As of 2021, it has about 8,900 inhabitants. It straddles the Lusatian Neisse river between Liberec and Jablonec nad Nisou, around 3.5 km south-east of Liberec city centre.

Leopold Ružička was a Croatian-Swiss scientist and joint winner of the 1939 Nobel Prize in Chemistry "for his work on polymethylenes and higher terpenes" "including the first chemical synthesis of male sex hormones." He worked most of his life in Switzerland, and received eight doctorates honoris causa in science, medicine, and law; seven prizes and medals; and twenty-four honorary memberships in chemical, biochemical, and other scientific societies.

Vladimir Prelog was a Croatian-Swiss organic chemist who received the 1975 Nobel Prize in chemistry for his research into the stereochemistry of organic molecules and reactions. Prelog was born, and spent his infancy, in Sarajevo, and youth in Zagreb, Osijek and Prague. He later lived and worked in Prague, Zagreb and Zürich.

Copper(II) sulfate is an inorganic compound with the chemical formula CuSO4. It forms hydrates CuSO4·nH2O, where n can range from 1 to 7. The pentahydrate (n = 5), a bright blue crystal, is the most commonly encountered hydrate of copper(II) sulfate, while its anhydrous form is white. Older names for the pentahydrate include blue vitriol, bluestone, vitriol of copper, and Roman vitriol. It exothermically dissolves in water to give the aquo complex [Cu(H2O)6]2+, which has octahedral molecular geometry. The structure of the solid pentahydrate reveals a polymeric structure wherein copper is again octahedral but bound to four water ligands. The Cu(II)(H2O)4 centers are interconnected by sulfate anions to form chains.

Karl Waldemar Ziegler was a German chemist who won the Nobel Prize in Chemistry in 1963, with Giulio Natta, for work on polymers. The Nobel Committee recognized his "excellent work on organometallic compounds [which]...led to new polymerization reactions and ... paved the way for new and highly useful industrial processes". He is also known for his work involving free-radicals, many-membered rings, and organometallic compounds, as well as the development of Ziegler–Natta catalyst. One of many awards Ziegler received was the Werner von Siemens Ring in 1960 jointly with Otto Bayer and Walter Reppe, for expanding the scientific knowledge of and the technical development of new synthetic materials.

Prof Paul Sabatier FRS(For) HFRSE was a French chemist, born in Carcassonne. In 1912, Sabatier was awarded the Nobel Prize in Chemistry along with Victor Grignard. Sabatier was honoured for his work improving the hydrogenation of organic species in the presence of metals.

Paul Karrer was a Swiss organic chemist best known for his research on vitamins. He and Norman Haworth won the Nobel Prize for Chemistry in 1937.
Iron(II) chloride, also known as ferrous chloride, is the chemical compound of formula FeCl2. It is a paramagnetic solid with a high melting point. The compound is white, but typical samples are often off-white. FeCl2 crystallizes from water as the greenish tetrahydrate, which is the form that is most commonly encountered in commerce and the laboratory. There is also a dihydrate. The compound is highly soluble in water, giving pale green solutions.
Jack David Dunitz FRS was a British chemist and widely known chemical crystallographer. He was Professor of Chemical Crystallography at the ETH Zurich from 1957 until his official retirement in 1990. He held Visiting Professorships in the United States, Israel, Japan, Canada, Spain and the United Kingdom.
Friedrich Adolf Paneth was an Austrian-born British chemist. Fleeing the Nazis, he escaped to Britain. He became a naturalized British citizen in 1939. After the war, Paneth returned to Germany to become director of the Max Planck Institute for Chemistry in 1953. He was considered the greatest authority of his time on volatile hydrides and also made important contributions to the study of the stratosphere.

Herman Francis Mark was an Austrian-American chemist regarded for his contributions to the development of polymer science. Mark's X-ray diffraction work on the molecular structure of fibers provided important evidence for the macromolecular theory of polymer structure. Together with Houwink he formulated an equation, now called the Mark–Houwink or Mark–Houwink–Sakurada equation, describing the dependence of the intrinsic viscosity of a polymer on its relative molecular mass. He was a long-time faculty at Polytechnic Institute of Brooklyn. In 1946, he established the Journal of Polymer Science.
Henri Boris Kagan is currently an emeritus professor at the Université Paris-Sud in France. He is widely recognized as a pioneer in the field of asymmetric catalysis. His discoveries have had far-reaching impacts on the pharmaceutical industry.
In organic chemistry, the Baudisch reaction is a process for the synthesis of nitrosophenols using metal ions. Although the products are of limited value, the reaction is of historical interest as an example of metal-promoted functionalization of aromatic substrates.

Albert Jakob Eschenmoser (5 August 1925 – 14 July 2023) was a Swiss organic chemist, best known for his work on the synthesis of complex heterocyclic natural compounds, most notably vitamin B12. In addition to his significant contributions to the field of organic synthesis, Eschenmoser pioneered work in the Origins of Life (OoL) field with work on the synthetic pathways of artificial nucleic acids. Before retiring in 2009, Eschenmoser held tenured teaching positions at the ETH Zurich and The Skaggs Institute for Chemical Biology at The Scripps Research Institute in La Jolla, California as well as visiting professorships at the University of Chicago, Cambridge University, and Harvard.
Selenium hexafluoride is the inorganic compound with the formula SeF6. It is a very toxic colourless gas described as having a "repulsive" odor. It is not widely encountered and has no commercial applications.

Nikolai Semyonovich Kurnakov was a Russian chemist, who is internationally recognized as the originator of physicochemical analysis. He also was one of the principal founders of the platinum industry in the Soviet Union. A chemical reaction that he pioneered, known as the Kurnakov test, is still used to differentiate cis from trans isomers of divalent platinum and is his best-known contribution to coordination chemistry.

Marjorie Constance Caserio was an English chemist. In 1975, she was awarded the Garvan Medal by the American Chemical Society.
Andreas Pfaltz is a Swiss chemist known for his work in the area of coordination chemistry and catalysis.
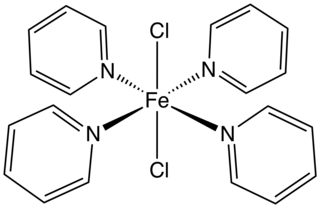
Dichlorotetrakis(pyridine)iron(II) is the coordination complex with the formula FeCl2(pyridine)4. A yellow solid, it is a prominent example of a transition metal pyridine complex. It is used as an anhydrous precursor to other iron complexes and catalysts. According to X-ray crystallography, the chloride ligands are mutually trans. The complex has a high spin configuration. A monohydrate as well as several related complexes are known, e.g. CoCl2(pyridine)4 and NiCl2(pyridine)4. It is prepared by treating ferrous chloride with an excess of pyridine.












