An ylide or ylid is a neutral dipolar molecule containing a formally negatively charged atom (usually a carbanion) directly attached to a heteroatom with a formal positive charge (usually nitrogen, phosphorus or sulfur), and in which both atoms have full octets of electrons. The result can be viewed as a structure in which two adjacent atoms are connected by both a covalent and an ionic bond; normally written X+–Y−. Ylides are thus 1,2-dipolar compounds, and a subclass of zwitterions. They appear in organic chemistry as reagents or reactive intermediates.

In organometallic chemistry, organolithium reagents are chemical compounds that contain carbon–lithium (C–Li) bonds. These reagents are important in organic synthesis, and are frequently used to transfer the organic group or the lithium atom to the substrates in synthetic steps, through nucleophilic addition or simple deprotonation. Organolithium reagents are used in industry as an initiator for anionic polymerization, which leads to the production of various elastomers. They have also been applied in asymmetric synthesis in the pharmaceutical industry. Due to the large difference in electronegativity between the carbon atom and the lithium atom, the C−Li bond is highly ionic. Owing to the polar nature of the C−Li bond, organolithium reagents are good nucleophiles and strong bases. For laboratory organic synthesis, many organolithium reagents are commercially available in solution form. These reagents are highly reactive, and are sometimes pyrophoric.

In organic chemistry, an imine is a functional group or organic compound containing a carbon–nitrogen double bond. The nitrogen atom can be attached to a hydrogen or an organic group (R). The carbon atom has two additional single bonds. Imines are common in synthetic and naturally occurring compounds and they participate in many reactions.
A 1,2-Wittig rearrangement is a categorization of chemical reactions in organic chemistry, and consists of a 1,2-rearrangement of an ether with an alkyllithium compound. The reaction is named for Nobel Prize winning chemist Georg Wittig.
The Wittig reaction or Wittig olefination is a chemical reaction of an aldehyde or ketone with a triphenyl phosphonium ylide called a Wittig reagent. Wittig reactions are most commonly used to convert aldehydes and ketones to alkenes. Most often, the Wittig reaction is used to introduce a methylene group using methylenetriphenylphosphorane (Ph3P=CH2). Using this reagent, even a sterically hindered ketone such as camphor can be converted to its methylene derivative.
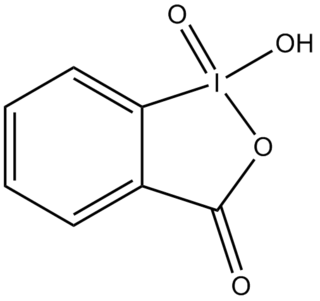
2-Iodoxybenzoic acid (IBX) is an organic compound used in organic synthesis as an oxidizing agent. This periodinane is especially suited to oxidize alcohols to aldehydes. IBX is most often prepared from 2-iodobenzoic acid and a strong oxidant such as potassium bromate and sulfuric acid, or more commonly, oxone. One of the main drawbacks of IBX is its limited solubility; IBX is insoluble in many common organic solvents. IBX is an impact- and heat-sensitive explosive (>200°C). Commercial IBX is stabilized by carboxylic acids such as benzoic acid and isophthalic acid.

The Jacobsen epoxidation, sometimes also referred to as Jacobsen-Katsuki epoxidation is a chemical reaction which allows enantioselective epoxidation of unfunctionalized alkyl- and aryl- substituted alkenes. It is complementary to the Sharpless epoxidation (used to form epoxides from the double bond in allylic alcohols). The Jacobsen epoxidation gains its stereoselectivity from a C2 symmetric manganese(III) salen-like ligand, which is used in catalytic amounts. The manganese atom transfers an oxygen atom from chlorine bleach or similar oxidant. The reaction takes its name from its inventor, Eric Jacobsen, with Tsutomu Katsuki sometimes being included. Chiral-directing catalysts are useful to organic chemists trying to control the stereochemistry of biologically active compounds and develop enantiopure drugs.

Phenyllithium is an organometallic agent with the empirical formula C6H5Li. It is most commonly used as a metalating agent in organic syntheses and a substitute for Grignard reagents for introducing phenyl groups in organic syntheses. Crystalline phenyllithium is colorless; however, solutions of phenyllithium are various shades of brown or red depending on the solvent used and the impurities present in the solute.

The Dakin oxidation (or Dakin reaction) is an organic redox reaction in which an ortho- or para-hydroxylated phenyl aldehyde (2-hydroxybenzaldehyde or 4-hydroxybenzaldehyde) or ketone reacts with hydrogen peroxide (H2O2) in base to form a benzenediol and a carboxylate. Overall, the carbonyl group is oxidised, whereas the H2O2 is reduced.
The Julia olefination (also known as the Julia–Lythgoe olefination) is the chemical reaction used in organic chemistry of phenyl sulfones (1) with aldehydes (or ketones) to give alkenes (olefins)(3) after alcohol functionalization and reductive elimination using sodium amalgam or SmI2. The reaction is named after the French chemist Marc Julia.
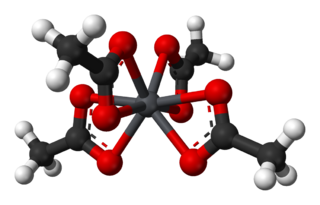
Lead(IV) acetate or lead tetraacetate is an metalorganic compound with chemical formula Pb(C2H3O2)4. It is a colorless solid that is soluble in nonpolar, organic solvents, indicating that it is not a salt. It is degraded by moisture and is typically stored with additional acetic acid. The compound is used in organic synthesis.
Deoxygenation is a chemical reaction involving the removal of oxygen atoms from a molecule. The term also refers to the removal of molecular oxygen (O2) from gases and solvents, a step in air-free technique and gas purifiers. As applied to organic compounds, deoxygenation is a component of fuels production as well a type of reaction employed in organic synthesis, e.g. of pharmaceuticals.
The Stieglitz rearrangement is a rearrangement reaction in organic chemistry which is named after the American chemist Julius Stieglitz (1867–1937) and was first investigated by him and Paul Nicholas Leech in 1913. It describes the 1,2-rearrangement of trityl amine derivatives to triaryl imines. It is comparable to a Beckmann rearrangement which also involves a substitution at a nitrogen atom through a carbon to nitrogen shift. As an example, triaryl hydroxylamines can undergo a Stieglitz rearrangement by dehydration and the shift of a phenyl group after activation with phosphorus pentachloride to yield the respective triaryl imine, a Schiff base.
Carbon satellites in physics and spectroscopy, are small peaks that can be seen shouldering the main peaks in the nuclear magnetic resonance (NMR) spectrum. These peaks can occur in the NMR spectrum of any NMR active atom where those atoms adjoin a carbon atom. However, Carbon satellites are most often encountered in proton NMR.
The [2,3]-Wittig rearrangement is the transformation of an allylic ether into a homoallylic alcohol via a concerted, pericyclic process. Because the reaction is concerted, it exhibits a high degree of stereocontrol, and can be employed early in a synthetic route to establish stereochemistry. The Wittig rearrangement requires strongly basic conditions, however, as a carbanion intermediate is essential. [1,2]-Wittig rearrangement is a competitive process.
The Criegee oxidation is a glycol cleavage reaction in which vicinal diols are oxidized to form ketones and aldehydes using lead tetraacetate. It is analogous to the use of periodate but uses a milder oxidant. This oxidation was discovered by Rudolf Criegee and coworkers and first reported in 1931 using ethylene glycol as the substrate.
A phosphetane is a 4-membered organophosphorus heterocycle. The parent phosphetane molecule, which has the formula C3H7P, is one atom larger than phosphiranes, one smaller than phospholes, and is the heavy-atom analogue of azetidines. The first known phosphetane synthesis was reported in 1957 by Kosolapoff and Struck, but the method was both inefficient and hard to reproduce, with yields rarely exceeding 1%. A far more efficient method was reported in 1962 by McBride, whose method allowed for the first studies into the physical and chemical properties of phosphetanes. Phosphetanes are a well understood class of molecules that have found broad applications as chemical building blocks, reagents for organic/inorganic synthesis, and ligands in coordination chemistry.
The Blum–Ittah aziridine synthesis, also known as the Blum–Ittah-Shahak aziridine synthesis or simply the Blum aziridine synthesis is a name reaction of organic chemistry, for the generation of aziridines from oxiranes.
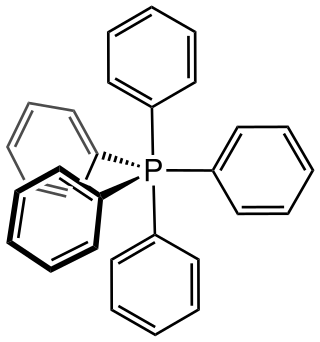
Pentaphenylphosphorus is an organic phosphorane containing five phenyl groups connected to a central phosphorus atom. The phosphorus atom is considered to be in the +5 oxidation state. The chemical formula could be written as P(C6H5)5 or Ph5P, where Ph represents the phenyl group. It was discovered and reported in 1949 by Georg Wittig.
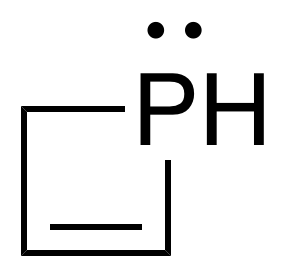
A phosphetene is an unsaturated four-membered organophosphorus heterocycle containing one phosphorus atom. It is a heavier analog of an azetine, or dihydroazete. The first synthesis of a stable, isolable phosphetene was reported in 1985 by Mathey et al. via ring expansion of a phosphirene-metal carbonyl complex. Since then, other synthesis routes for phosphetenes have been developed such as cyclization of phosphabutadienes, [2 + 2] cycloaddition, intramolecular arrangement, addition, and through organometallic intermediates.













