Angiogenesis is the physiological process through which new blood vessels form from pre-existing vessels, formed in the earlier stage of vasculogenesis. Angiogenesis continues the growth of the vasculature mainly by processes of sprouting and splitting, but processes such as coalescent angiogenesis, vessel elongation and vessel cooption also play a role. Vasculogenesis is the embryonic formation of endothelial cells from mesoderm cell precursors, and from neovascularization, although discussions are not always precise. The first vessels in the developing embryo form through vasculogenesis, after which angiogenesis is responsible for most, if not all, blood vessel growth during development and in disease.
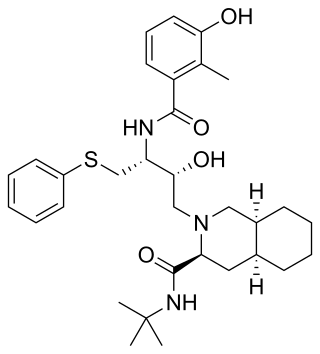
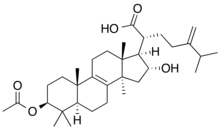
Nelfinavir, sold under the brand name Viracept, is an antiretroviral medication used in the treatment of HIV/AIDS. Nelfinavir belongs to the class of drugs known as protease inhibitors (PIs) and like other PIs is almost always used in combination with other antiretroviral drugs.
An angiogenesis inhibitor is a substance that inhibits the growth of new blood vessels (angiogenesis). Some angiogenesis inhibitors are endogenous and a normal part of the body's control and others are obtained exogenously through pharmaceutical drugs or diet.

Hepatocyte growth factor receptor is a protein that in humans is encoded by the MET gene. The protein possesses tyrosine kinase activity. The primary single chain precursor protein is post-translationally cleaved to produce the alpha and beta subunits, which are disulfide linked to form the mature receptor.
The epoxyeicosatrienoic acids or EETs are signaling molecules formed within various types of cells by the metabolism of arachidonic acid by a specific subset of cytochrome P450 enzymes termed cytochrome P450 epoxygenases. These nonclassic eicosanoids are generally short-lived, being rapidly converted from epoxides to less active or inactive dihydroxy-eicosatrienoic acids (diHETrEs) by a widely distributed cellular enzyme, soluble epoxide hydrolase (sEH), also termed epoxide hydrolase 2. The EETs consequently function as transiently acting, short-range hormones; that is, they work locally to regulate the function of the cells that produce them or of nearby cells. The EETs have been most studied in animal models where they show the ability to lower blood pressure possibly by a) stimulating arterial vasorelaxation and b) inhibiting the kidney's retention of salts and water to decrease intravascular blood volume. In these models, EETs prevent arterial occlusive diseases such as heart attacks and brain strokes not only by their anti-hypertension action but possibly also by their anti-inflammatory effects on blood vessels, their inhibition of platelet activation and thereby blood clotting, and/or their promotion of pro-fibrinolytic removal of blood clots. With respect to their effects on the heart, the EETs are often termed cardio-protective. Beyond these cardiovascular actions that may prevent various cardiovascular diseases, studies have implicated the EETs in the pathological growth of certain types of cancer and in the physiological and possibly pathological perception of neuropathic pain. While studies to date imply that the EETs, EET-forming epoxygenases, and EET-inactivating sEH can be manipulated to control a wide range of human diseases, clinical studies have yet to prove this. Determination of the role of the EETS in human diseases is made particularly difficult because of the large number of EET-forming epoxygenases, large number of epoxygenase substrates other than arachidonic acid, and the large number of activities, some of which may be pathological or injurious, that the EETs possess.

Proline-, glutamic acid- and leucine-rich protein 1 (PELP1) also known as modulator of non-genomic activity of estrogen receptor (MNAR) and transcription factor HMX3 is a protein that in humans is encoded by the PELP1 gene. is a transcriptional corepressor for nuclear receptors such as glucocorticoid receptors and a coactivator for estrogen receptors.

Interleukin 24 (IL-24) is a protein in the interleukin family, a type of cytokine signaling molecule in the immune system. In humans, this protein is encoded by the IL24 gene.

Honokiol is a lignan isolated from the bark, seed cones, and leaves of trees belonging to the genus Magnolia. It has been identified as one of the chemical compounds in some traditional eastern herbal medicines along with magnolol, 4-O-methylhonokiol, and obovatol.

Wolfiporia extensa, commonly known as hoelen, poria, tuckahoe, China root, fu ling, or matsuhodo, is a fungus in the family Polyporaceae. It is a wood-decay fungus but has a subterranean growth habit. It is notable in the development of a large, long-lasting underground sclerotium that resembles a small coconut. This sclerotium, known as Tuckahoe or fu-ling, is not the same as the true tuckahoe used as Indian bread by Native Americans, which is the arrow arum, Peltandra virginica, a flowering tuberous plant in the arum family.

Krüppel-like factor 4 is a member of the KLF family of zinc finger transcription factors, which belongs to the relatively large family of SP1-like transcription factors. KLF4 is involved in the regulation of proliferation, differentiation, apoptosis and somatic cell reprogramming. Evidence also suggests that KLF4 is a tumor suppressor in certain cancers, including colorectal cancer. It has three C2H2-zinc fingers at its carboxyl terminus that are closely related to another KLF, KLF2. It has two nuclear localization sequences that signals it to localize to the nucleus. In embryonic stem cells (ESCs), KLF4 has been demonstrated to be a good indicator of stem-like capacity. It is suggested that the same is true in mesenchymal stem cells (MSCs).

Pigment epithelium-derived factor (PEDF) also known as serpin F1 (SERPINF1), is a multifunctional secreted protein that has anti-angiogenic, anti-tumorigenic, and neurotrophic functions. Found in vertebrates, this 50 kDa protein is being researched as a therapeutic candidate for treatment of such conditions as choroidal neovascularization, heart disease, and cancer. In humans, pigment epithelium-derived factor is encoded by the SERPINF1 gene.

Glypican-3 is a protein that, in humans, is encoded by the GPC3 gene. The GPC3 gene is located on human X chromosome (Xq26) where the most common gene encodes a 70-kDa core protein with 580 amino acids. Three variants have been detected that encode alternatively spliced forms termed Isoforms 1 (NP_001158089), Isoform 3 (NP_001158090) and Isoform 4 (NP_001158091).

Zinc transporter SLC39A7 (ZIP7), also known as solute carrier family 39 member 7, is a transmembrane protein that in humans is encoded by the SLC39A7 gene. It belongs to the ZIP family, which consists of 14 proteins that transport zinc into the cytoplasm. Its primary role is to control the transport of zinc from the ER and Golgi apparatus to the cytoplasm. It also plays a role in glucose metabolism. Its structure consists of helices that bind to zinc in a binuclear metal center. Its fruit fly orthologue is Catsup.

Protocatechuic acid (PCA) is a dihydroxybenzoic acid, a type of phenolic acid. It is a major metabolite of antioxidant polyphenols found in green tea. It has mixed effects on normal and cancer cells in in vitro and in vivo studies.
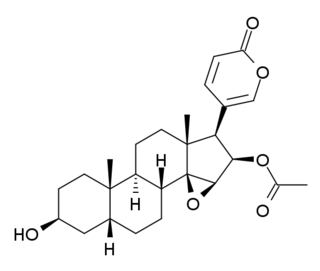
Cinobufagin is a cardiotoxic bufanolide steroid secreted by the Asiatic toad Bufo gargarizans. It has similar effects to digitalis and is used in traditional Chinese medicine.

Scutellarin is a flavone, a type of phenolic chemical compound. It can be found in the Asian "barbed skullcap" Scutellaria barbata and the north American plant S. lateriflora both of which have been used in traditional medicine. The compound is found only in trace amounts in the "Chinese skullcap" Scutellaria baicalensis, another plant used in traditional Chinese medicine.
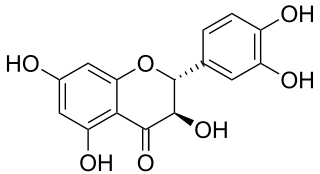
Taxifolin (5,7,3',4'-flavan-on-ol), also known as dihydroquercetin, belongs to the subclass flavanonols in the flavonoids, which in turn is a class of polyphenols. It is extracted from plants such as Siberian larch and milk thistle.

Sonodynamic therapy (SDT) is a noninvasive treatment, often used for tumor irradiation, that utilizes a sonosensitizer and the deep penetration of ultrasound to treat lesions of varying depths by reducing target cell number and preventing future tumor growth. Many existing cancer treatment strategies cause systemic toxicity or cannot penetrate tissue deep enough to reach the entire tumor; however, emerging ultrasound stimulated therapies could offer an alternative to these treatments with their increased efficiency, greater penetration depth, and reduced side effects. Sonodynamic therapy could be used to treat cancers and other diseases, such as atherosclerosis, and diminish the risk associated with other treatment strategies since it induces cytotoxic effects only when externally stimulated by ultrasound and only at the cancerous region, as opposed to the systemic administration of chemotherapy drugs.
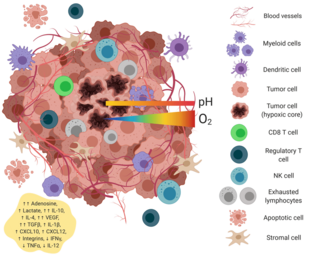
The tumor microenvironment is a complex ecosystem surrounding a tumor, composed of cancer cells, stromal tissue and the extracellular matrix. Mutual interaction between cancer cells and the different components of the tumor microenvironment support its growth and invasion in healthy tissues which correlates with tumor resistance to current treatments and poor prognosis. The tumor microenvironment is in constant change because of the tumor's ability to influence the microenvironment by releasing extracellular signals, promoting tumor angiogenesis and inducing peripheral immune tolerance, while the immune cells in the microenvironment can affect the growth and evolution of cancerous cells.
Exosomes are small vesicles secreted by cells that play a crucial role in intercellular communication. They contain a variety of biomolecules, including proteins, nucleic acids and lipids, which can be transferred between cells to modulate cellular processes. Exosomes have been increasingly acknowledged as promising therapeutic tool and delivery platforms due to unique biological properties.
- Biocompatibility: Exosomes are naturally occurring particles in body, which makes them highly biocompatible and less likely to activate immune response.
- Targeting ability: Exosomes are assembled to express specific proteins or peptides, allowing them to target specific cells or tissues.
- Natural cargo carries: Exosomes can naturally transport a variety of biomolecules, including proteins, RNA and DNA, which can be used for therapeutic purposes.