In organic chemistry, an imine is a functional group or organic compound containing a carbon–nitrogen double bond. The nitrogen atom can be attached to a hydrogen or an organic group (R). The carbon atom has two additional single bonds. Imines are common in synthetic and naturally occurring compounds and they participate in many reactions.
In organic chemistry, an acyl chloride is an organic compound with the functional group −C(=O)Cl. Their formula is usually written R−COCl, where R is a side chain. They are reactive derivatives of carboxylic acids. A specific example of an acyl chloride is acetyl chloride, CH3COCl. Acyl chlorides are the most important subset of acyl halides.

Hydroiodic acid is a colorless liquid. It is an aqueous solution of hydrogen iodide with the chemical formula HI(aq). It is a strong acid, in which hydrogen iodide is ionized completely in an aqueous solution. Concentrated aqueous solutions of hydrogen iodide are usually 48% to 57% HI by mass.

In organic chemistry, nitration is a general class of chemical processes for the introduction of a nitro group into an organic compound. The term also is applied incorrectly to the different process of forming nitrate esters between alcohols and nitric acid. The difference between the resulting molecular structures of nitro compounds and nitrates is that the nitrogen atom in nitro compounds is directly bonded to a non-oxygen atom, whereas in nitrate esters, the nitrogen is bonded to an oxygen atom that in turn usually is bonded to a carbon atom.

In organic chemistry, nitro compounds are organic compounds that contain one or more nitro functional groups. The nitro group is one of the most common explosophores used globally. The nitro group is also strongly electron-withdrawing. Because of this property, C−H bonds alpha (adjacent) to the nitro group can be acidic. For similar reasons, the presence of nitro groups in aromatic compounds retards electrophilic aromatic substitution but facilitates nucleophilic aromatic substitution. Nitro groups are rarely found in nature. They are almost invariably produced by nitration reactions starting with nitric acid.

Azo compounds are organic compounds bearing the functional group diazenyl.

In organic and inorganic chemistry, nitroamines or nitramides are chemical compounds with the general chemical structure R1R2N−NO2. They consist of a nitro group bonded to the nitrogen of an amine. The R groups can be any group, typically hydrogen and organyl. An example of inorganic nitroamine is chloronitroamine or chloro(nitro)amine Cl−NH−NO2. The parent inorganic compound, where both R substituents are hydrogen, is nitramide or nitroamine, H2N−NO2.
In organic chemistry, the Mannich reaction is a three-component organic reaction that involves the amino alkylation of an acidic proton next to a carbonyl functional group by formaldehyde and a primary or secondary amine or ammonia. The final product is a β-amino-carbonyl compound also known as a Mannich base. Reactions between aldimines and α-methylene carbonyls are also considered Mannich reactions because these imines form between amines and aldehydes. The reaction is named after Carl Mannich.
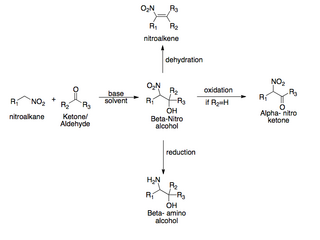
The Henry reaction is a classic carbon–carbon bond formation reaction in organic chemistry. Discovered in 1895 by the Belgian chemist Louis Henry (1834–1913), it is the combination of a nitroalkane and an aldehyde or ketone in the presence of a base to form β-nitro alcohols. This type of reaction is also referred to as a nitroaldol reaction. It is nearly analogous to the aldol reaction that had been discovered 23 years prior that couples two carbonyl compounds to form β-hydroxy carbonyl compounds known as "aldols". The Henry reaction is a useful technique in the area of organic chemistry due to the synthetic utility of its corresponding products, as they can be easily converted to other useful synthetic intermediates. These conversions include subsequent dehydration to yield nitroalkenes, oxidation of the secondary alcohol to yield α-nitro ketones, or reduction of the nitro group to yield β-amino alcohols.
Adams' catalyst, also known as platinum dioxide, is usually represented as platinum(IV) oxide hydrate, PtO2•H2O. It is a catalyst for hydrogenation and hydrogenolysis in organic synthesis. This dark brown powder is commercially available. The oxide itself is not an active catalyst, but it becomes active after exposure to hydrogen whereupon it converts to platinum black, which is responsible for reactions.
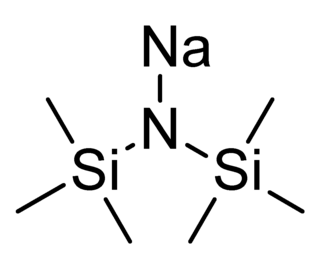
Sodium bis(trimethylsilyl)amide is the organosilicon compound with the formula NaN(Si 3)2. This species, usually called NaHMDS, is a strong base used for deprotonation reactions or base-catalyzed reactions. Its advantages are that it is commercially available as a solid and it is soluble not only in ethers, such as THF or diethyl ether, but also in aromatic solvents, like benzene and toluene by virtue of the lipophilic TMS groups.

Metiamide is a histamine H2 receptor antagonist developed from another H2 antagonist, burimamide. It was an intermediate compound in the development of the successful anti-ulcer drug cimetidine (Tagamet).
Benzyl chloride, or α-chlorotoluene, is an organic compound with the formula C6H5CH2Cl. This colorless liquid is a reactive organochlorine compound that is a widely used chemical building block.

In organic chemistry, nitroso refers to a functional group in which the nitric oxide group is attached to an organic moiety. As such, various nitroso groups can be categorized as C-nitroso compounds, S-nitroso compounds, N-nitroso compounds, and O-nitroso compounds.
In organic chemistry, the Nef reaction is an organic reaction describing the acid hydrolysis of a salt of a primary or secondary nitroalkane to an aldehyde or a ketone and nitrous oxide. The reaction has been the subject of several literature reviews.
The reduction of nitro compounds are chemical reactions of wide interest in organic chemistry. The conversion can be effected by many reagents. The nitro group was one of the first functional groups to be reduced. Alkyl and aryl nitro compounds behave differently. Most useful is the reduction of aryl nitro compounds.
The Béchamp reduction is a chemical reaction that converts aromatic nitro compounds to their corresponding anilines using iron as the reductant:

Atevirdine is a non-nucleoside reverse transcriptase inhibitor that has been studied for the treatment of HIV.

The Quelet reaction is an organic coupling reaction in which a phenolic ether reacts with an aliphatic aldehyde to generate an α-chloroalkyl derivative. The Quelet reaction is an example of a larger class of reaction, electrophilic aromatic substitution. The reaction is named after its creator R. Quelet, who first reported the reaction in 1932, and is similar to the Blanc chloromethylation process.
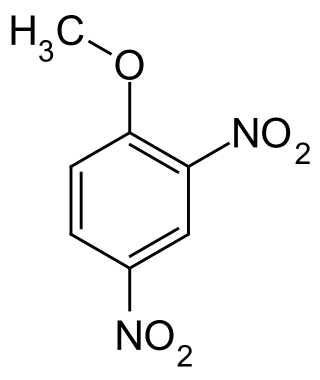
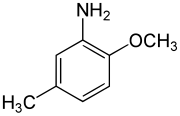
2,4-Dinitroanisole (DNAN) is a low sensitivity organic compound. It has an anisole (methoxybenzene) core, with two nitro groups (–NO2) attached.