
The rhinovirus is the most common viral infectious agent in humans and is the predominant cause of the common cold. Rhinovirus infection proliferates in temperatures of 33–35 °C (91–95 °F), the temperatures found in the nose. Rhinoviruses belong to the genus Enterovirus in the family Picornaviridae.
Coxsackie B4 virus are enteroviruses that belong to the Picornaviridae family. These viruses can be found worldwide. They are positive-sense, single-stranded, non-enveloped RNA viruses with icosahedral geometry. Coxsackieviruses have two groups, A and B, each associated with different diseases. Coxsackievirus group A is known for causing hand-foot-and-mouth diseases while Group B, which contains six serotypes, can cause a varying range of symptoms like gastrointestinal distress myocarditis. Coxsackievirus B4 has a cell tropism for natural killer cells and pancreatic islet cells. Infection can lead to beta cell apoptosis which increases the risk of insulitis.

Picornaviruses are a group of related nonenveloped RNA viruses which infect vertebrates including fish, mammals, and birds. They are viruses that represent a large family of small, positive-sense, single-stranded RNA viruses with a 30 nm icosahedral capsid. The viruses in this family can cause a range of diseases including the common cold, poliomyelitis, meningitis, hepatitis, and paralysis.
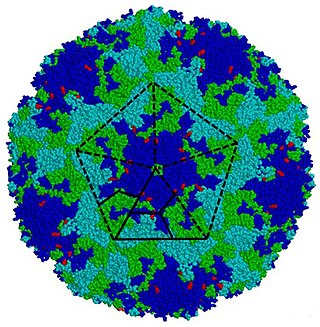
Enterovirus is a genus of positive-sense single-stranded RNA viruses associated with several human and mammalian diseases. Enteroviruses are named by their transmission-route through the intestine.

Aphthovirus is a viral genus of the family Picornaviridae. Aphthoviruses infect split-hooved animals, and include the causative agent of foot-and-mouth disease, Foot-and-mouth disease virus (FMDV). There are seven FMDV serotypes: A, O, C, SAT 1, SAT 2, SAT 3 and Asia 1, and four non-FMDV serotypes belonging to three additional species Bovine rhinitis A virus (BRAV), Bovine rhinitis B virus (BRBV) and Equine rhinitis A virus (ERAV).

Cardiovirus are a group of viruses within order Picornavirales, family Picornaviridae. Vertebrates serve as natural hosts for these viruses.

Dependoparvovirus is a genus in the subfamily Parvovirinae of the virus family Parvoviridae; they are Group II viruses according to the Baltimore classification. Some dependoparvoviruses are also known as adeno-associated viruses because they cannot replicate productively in their host cell without the cell being coinfected by a helper virus such as an adenovirus, a herpesvirus, or a vaccinia virus.

Mengovirus, also known as Columbia SK virus, mouse Elberfield virus, and Encephalomyocarditisvirus (EMCV), belongs to the genus Cardiovirus which is a member of the Picornaviridae. Its genome is a single stranded positive-sense RNA molecule, making the Mengoviruses a class IV virus under the Baltimore classification system. The genome is approximately 8400nt in length, and has 5’ VG protein and a 3’ polyadenine tail. Mengovirus was isolated by George W. A. Dick in 1948, in the Mengo district of Entebbe in Uganda, from a captive rhesus monkey that had developed hind limb paralysis.
Erbovirus is a genus of viruses in the order Picornavirales, in the family Picornaviridae. Horses serve as natural hosts. There is only one species in this genus: Erbovirus A. Diseases associated with this genus include: upper respiratory tract disease with viremia and fecal shedding. Viruses belonging to the genus Erbovirus have been isolated in horses with acute upper febrile respiratory disease. The structure of the Erbovirus virion is icosahedral, having a diameter of 27–30 nm.

Pleconaril (Picovir) is an antiviral drug that was being developed by Schering-Plough for prevention of asthma exacerbations and common cold symptoms in patients exposed to picornavirus respiratory infections. Pleconaril, administered either orally or intranasally, is active against viruses in the Picornaviridae family, including Enterovirus and Rhinovirus. It has shown useful activity against the dangerous enterovirus D68.

This family represents the internal ribosome entry site (IRES) of the hepatitis A virus. HAV IRES is a 450 nucleotide long sequence located in the 735 nt long 5’ UTR of Hepatitis A viral RNA genome. IRES elements allow cap and end-independent translation of mRNA in the host cell. The IRES achieves this by mediating the internal initiation of translation by recruiting a ribosomal 40S pre-initiation complex directly to the initiation codon and eliminates the requirement for eukaryotic initiation factor, eIF4F.
Parechovirus is a genus of viruses in the family Picornaviridae. Humans, ferrets, and various rodents serve as natural hosts. The genus currently consists of six accepted species. Human parechoviruses may cause gastrointestinal or respiratory illness in infants, and they have been implicated in cases of myocarditis and encephalitis.

Vincent R. Racaniello is a Higgins Professor in the Department of Microbiology and Immunology at Columbia University's College of Physicians and Surgeons. He is a co-author of a textbook on virology, Principles of Virology.

Enterovirus E is a picornavirus of the genus Enterovirus. The virus may also be referred to as enteric cytopathic bovine orphan virus (ECBO). It is endemic in cattle populations worldwide, and although normally fairly nonpathogenic, it can cause reproductive, respiratory, or enteric disease – particularly when the animal is concurrently infected with another pathogen.
Cricket paralysis virus (CrPV) was initially discovered in Australian field crickets by Carl Reinganum and his colleagues at the Victorian Plant Research Institute. The paralytic disease spread rapidly through a breeding colony as well as through a laboratory population causing about 95% mortality. This was the first recorded isolate of the virus and is generally referred to as CrPVvic to distinguish it from subsequent isolates.
Bocaparvovirus is a genus of viruses in the subfamily Parvovirinae of the virus family Parvoviridae. Humans, cattle, and dogs serve as natural hosts. There are 28 species in this genus. Diseases associated with this genus include, in humans, acute respiratory illness, and in cattle, diarrhea and mild respiratory symptoms.

Picornain 3C is a protease found in picornaviruses, which cleaves peptide bonds of non-terminal sequences. Picornain 3C’s endopeptidase activity is primarily responsible for the catalytic process of selectively cleaving Gln-Gly bonds in the polyprotein of poliovirus and with substitution of Glu for Gln, and Ser or Thr for Gly in other picornaviruses. Picornain 3C are cysteine proteases related by amino acid sequence to trypsin-like serine proteases. Picornain 3C is encoded by enteroviruses, rhinoviruses, aphtoviruses and cardioviruses. These genera of picoviruses cause a wide range of infections in humans and mammals.

Pneumoviridae is a family of negative-strand RNA viruses in the order Mononegavirales. Humans, cattle, and rodents serve as natural hosts. Respiratory tract infections are associated with member viruses such as human respiratory syncytial virus. There are five species in the family which are divided between the genera Metapneumovirus and Orthopneumovirus. The family used to be considered as a sub-family of Paramyxoviridae, but has been reclassified as of 2016.
Triatoma virus (TrV) is a virus belonging to the insect virus family Dicistroviridae. Within this family, there are currently 3 genera and 15 species of virus. Triatoma virus belongs to the genus Cripavirus. It is non-enveloped and its genetic material is positive-sense, single-stranded RNA. The natural hosts of triatoma virus are invertebrates. TrV is a known pathogen to Triatoma infestans, the major vector of Chagas disease in Argentina which makes triatoma virus a major candidate for biological vector control as opposed to chemical insecticides. Triatoma virus was first discovered in 1984 when a survey of pathogens of triatomes was conducted in the hopes of finding potential biological control methods for T. infestans.

The black queen cell virus (BQCV) is a virus that infects honey bees, specifically Apis mellifera, Apis florea, and Apis dorsata. Infection of the latter two species is more recent and can be attributed to genetic similarity and geographical closeness. It is important to learn about this virus because it is one of the most common bee viruses and bees are the most important pollinators. The agricultural industry depends on the bee's pollination to increase its economic value.














