
Arnica montana, also known as wolf's bane, leopard's bane, mountain tobacco and mountain arnica, is a moderately toxic European flowering plant in the daisy family Asteraceae. It is noted for its large yellow flower head. The names "wolf's bane" and "leopard's bane" are also used for another plant, aconitum, which is extremely poisonous.

Bilobalide is a biologically active terpenic trilactone present in Ginkgo biloba.
Sesquiterpene lactones (SLs) are a class of sesquiterpenoids that contain a lactone ring. They are most often found in plants of the family Asteraceae. Other plant families with SLs are Umbelliferae and Magnoliaceae (magnolias). A collection of colorless, lipophilic solids, SLs are a rich source of drugs. They can be allergenic and toxic in grazing livestock causing severe neurological problems in horses. Some are also found in corals such as Maasella edwardsi.

Lactucin is a bitter substance that forms a white crystalline solid and belongs to the group of sesquiterpene lactones. It is found in some varieties of lettuce and is an ingredient of lactucarium. It has been shown to have analgesic and sedative properties. It has also shown some antimalarial effects. It is also found in dandelion coffee.

Lactucopicrin (Intybin) is a bitter substance that has a sedative and analgesic effect, acting on the central nervous system. It is a sesquiterpene lactone, and is a component of lactucarium, derived from the plant Lactuca virosa, as well as being found in some related plants such as Cichorium intybus. It is also found in dandelion coffee.

Parthenolide is a sesquiterpene lactone of the germacranolide class which occurs naturally in the plant feverfew, after which it is named, and in the closely related tansy. It is found in highest concentration in the flowers and fruit. Parthenolide's molecular structure depiction is often incorrect regarding the stereochemistry of the epoxide, although X-ray single crystal structures are available.

Sesquiterpenes are a class of terpenes that consist of three isoprene units and often have the molecular formula C15H25. Like monoterpenes, sesquiterpenes may be cyclic or contain rings, including many unique combinations. Biochemical modifications such as oxidation or rearrangement produce the related sesquiterpenoids.

Helenalin, or (-)-4-Hydroxy-4a,8-dimethyl-3,3a,4a,7a,8,9,9a-octahydroazuleno[6,5-b]furan-2,5-dione, is a toxic sesquiterpene lactone which can be found in several plants such as Arnica montana and Arnica chamissonis Helenalin is responsible for the toxicity of the Arnica spp. Although toxic, helenalin possesses some in vitro anti-inflammatory and anti-neoplastic effects. Helenalin can inhibit certain enzymes, such as 5-lipoxygenase and leukotriene C4 synthase. For this reason the compound or its derivatives may have potential medical applications.

Parthenium is a genus of North American annuals, biennials, perennials, subshrubs, and shrubs in the tribe Heliantheae within the family Asteraceae and subfamily Asteroideae.
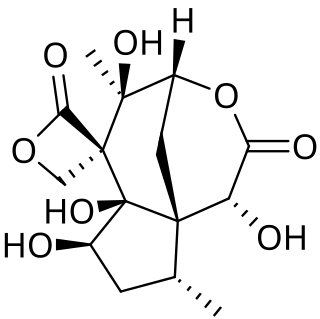
Anisatin is an extremely toxic, insecticidally active component of the shikimi plant. The lethal dose is 1 mg/kg (i.p.) in mice. Symptoms begin to appear about 1–6 hours after ingestion, beginning with gastrointestinal ailments, such as diarrhea, vomiting, and stomach pain, followed by nervous system excitation, seizures, loss of consciousness, and respiratory paralysis, which is the ultimate cause of death.

Absinthin is a naturally produced triterpene lactone from the plant Artemisia absinthium (Wormwood). It constitutes one of the most bitter chemical agents responsible for absinthe's distinct taste. The compound shows biological activity and has shown promise as an anti-inflammatory agent, and should not to be confused with thujone, a neurotoxin also found in Artemisia absinthium.

Merrilactone A is one of the four sesquiterpenes that were newly discovered from the fruit of Illicium merrillianum in 2000. Members of the genus Illicium include Chinese star anise, widely used as a spice for flavouring food and beverages, and also poisonous plants such as Japanese star anise. Chemical studies of Illicium have developed rapidly over the last 20 years, and merrilactone A has been shown to have neurotrophic activity in fetal rat cortical neuron cultures. This has led researchers to believe that Merrilactone A may hold therapeutic potential in the treatment of neuro-degenerative diseases such as Alzheimer's disease and Parkinson's disease.

(+)-Costunolide is a naturally occurring sesquiterpene lactone, first isolated in Saussurea costus roots in 1960. It is also found in lettuce.

Parthenium hysterophorus is a species of flowering plant in the family Asteraceae. It is native to the American tropics. Common names include Santa-Maria, Santa Maria feverfew, whitetop weed, and famine weed. In India, it is locally known as carrot grass, congress grass or gajar ghas or dhanura. It is a common invasive species in India, Australia, and parts of Africa.

Vernolepin is a sesquiterpene lactone isolated from the dried fruit of Vernonia amygdalina. It shows platelet anti-aggregating properties and is also an irreversible DNA polymerase inhibitor, hence may have antitumor properties.
Cynaropicrin is a sesquiterpene lactone of the guaianolide type found mainly in leaves of artichoke plants. It is one of the compounds that gives the artichoke its characteristic bitterness. It is found in artichoke leaves with an abundance of approximately 87 g/kg, but can hardly be found in other parts of the plant. Cynaropicrin makes up about 0.7% of leaf extracts of the artichoke. It exhibits a large diversity of bioactivities and shows properties such as anti-inflammatory, antifeedant and activation of bitter sensory receptors, but has not yet been used in medicine. Despite its pharmacologically beneficial properties, it can be toxic in higher doses. The compound has attracted attention in recent years as a potential anticancer drug.

Helenin is a phytochemical mixture found in many plant species, including the Inula helenium (elecampane) of the family Asteraceae. It is a mixture of two isomeric sesquiterpene lactones, alantolactone and isoalantolactone.

Arglabin is a sesquiterpene lactone belonging to the guaianolide subclass bearing a 5,7,5-tricyclic ring system which is known to inhibit farnesyl transferase. It is characterized by an epoxide on the cycloheptane as well as an exocyclic methylene group that is conjugated with the carbonyl of the lactone. Arglabin is extracted from Artemisia glabella, a species of wormwood, found in the Karaganda Region of Kazakhstan. Arglabin and its derivatives are biologically active and demonstrate promising antitumor activity and cytoxocity against varying tumor cell lines.

In organic chemistry, a guaianolide is a type of sesquiterpene lactone consisting of a gamma-lactone and either a cyclopentane or cyclopentene, both fused to a central cycloheptane or cycloheptene structure. There are two subclasses, structural isomers differing in the location that part of the lactone is bonded to the central ring, known as 6,12-guaianolides and 8,12-guaianolides.

Xanthatin, or (3aR,7S,8aS)-7-methyl-3-methylidene-6-[(E)-3-oxobut-1-enyl]-4,7,8,8a-tetrahydro-3aH-cyclohepta[b]furan-2-one (C15H18O3) is a major bioactive compound found in the leaves of the Xanthium strumarium (Asteracae) plant. It is classified as a natural sesquiterpene lactone. Xanthatin is believed to have anti-inflammatory, anti-tumour, anti-microbial, and anti-parasitic properties hence it is being researched for potential use in treatment of cancer and autoimmune diseases. While it has been used in traditional medicine for decades, its mechanisms and modern use haven’t been fully understood yet.


















