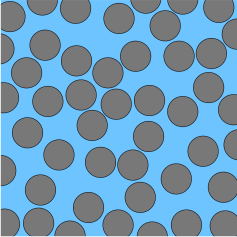
A colloid is a mixture in which one substance consisting of microscopically dispersed insoluble particles is suspended throughout another substance. However, some definitions specify that the particles must be dispersed in a liquid, and others extend the definition to include substances like aerosols and gels. The term colloidal suspension refers unambiguously to the overall mixture. A colloid has a dispersed phase and a continuous phase. The dispersed phase particles have a diameter of approximately 1 nanometre to 1 micrometre.

Electrophoresis is the motion of dispersed particles relative to a fluid under the influence of a spatially uniform electric field. Electrophoresis of positively charged particles (cations) is sometimes called cataphoresis, while electrophoresis of negatively charged particles (anions) is sometimes called anaphoresis.
Ultrafiltration (UF) is a variety of membrane filtration in which forces like pressure or concentration gradients lead to a separation through a semipermeable membrane. Suspended solids and solutes of high molecular weight are retained in the so-called retentate, while water and low molecular weight solutes pass through the membrane in the permeate (filtrate). This separation process is used in industry and research for purifying and concentrating macromolecular (103–106 Da) solutions, especially protein solutions.
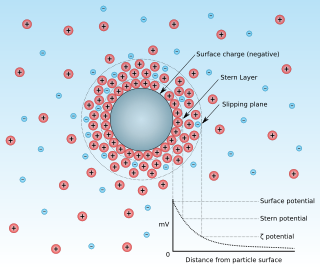
Zeta potential is the electrical potential at the slipping plane. This plane is the interface which separates mobile fluid from fluid that remains attached to the surface.
The DLVO theory explains the aggregation of aqueous dispersions quantitatively and describes the force between charged surfaces interacting through a liquid medium. It combines the effects of the van der Waals attraction and the electrostatic repulsion due to the so-called double layer of counterions. The electrostatic part of the DLVO interaction is computed in the mean field approximation in the limit of low surface potentials - that is when the potential energy of an elementary charge on the surface is much smaller than the thermal energy scale, . For two spheres of radius each having a charge separated by a center-to-center distance in a fluid of dielectric constant containing a concentration of monovalent ions, the electrostatic potential takes the form of a screened-Coulomb or Yukawa potential,
An artificial membrane, or synthetic membrane, is a synthetically created membrane which is usually intended for separation purposes in laboratory or in industry. Synthetic membranes have been successfully used for small and large-scale industrial processes since the middle of twentieth century. A wide variety of synthetic membranes is known. They can be produced from organic materials such as polymers and liquids, as well as inorganic materials. The most of commercially utilized synthetic membranes in separation industry are made of polymeric structures. They can be classified based on their surface chemistry, bulk structure, morphology, and production method. The chemical and physical properties of synthetic membranes and separated particles as well as a choice of driving force define a particular membrane separation process. The most commonly used driving forces of a membrane process in industry are pressure and concentration gradients. The respective membrane process is therefore known as filtration. Synthetic membranes utilized in a separation process can be of different geometry and of respective flow configuration. They can also be categorized based on their application and separation regime. The best known synthetic membrane separation processes include water purification, reverse osmosis, dehydrogenation of natural gas, removal of cell particles by microfiltration and ultrafiltration, removal of microorganisms from dairy products, and Dialysis.
Surface charge is a two-dimensional surface with non-zero electric charge. These electric charges are constrained on this 2-D surface, and surface charge density, measured in coulombs per square meter (C•m−2), is used to describe the charge distribution on the surface. The electric potential is continuous across a surface charge and the electric field is discontinuous, but not infinite; this is unless the surface charge consists of a dipole layer. In comparison, the potential and electric field both diverge at any point charge or linear charge.

Fouling is the accumulation of unwanted material on solid surfaces. The fouling materials can consist of either living organisms (biofouling) or a non-living substance. Fouling is usually distinguished from other surface-growth phenomena in that it occurs on a surface of a component, system, or plant performing a defined and useful function and that the fouling process impedes or interferes with this function.

Particle agglomeration refers to formation of assemblages in a suspension and represents a mechanism leading to the functional destabilization of colloidal systems. During this process, particles dispersed in the liquid phase stick to each other, and spontaneously form irregular particle assemblages, flocs, or agglomerates. This phenomenon is also referred to as coagulation or flocculation and such a suspension is also called unstable. Particle agglomeration can be induced by adding salts or other chemicals referred to as coagulant or flocculant.
The Gibbs adsorption isotherm for multicomponent systems is an equation used to relate the changes in concentration of a component in contact with a surface with changes in the surface tension, which results in a corresponding change in surface energy. For a binary system, the Gibbs adsorption equation in terms of surface excess is:
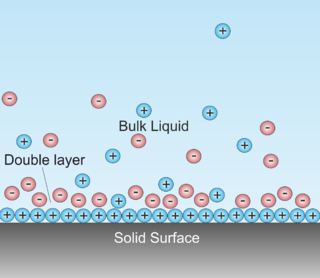
A double layer is a structure that appears on the surface of an object when it is exposed to a fluid. The object might be a solid particle, a gas bubble, a liquid droplet, or a porous body. The DL refers to two parallel layers of charge surrounding the object. The first layer, the surface charge, consists of ions adsorbed onto the object due to chemical interactions. The second layer is composed of ions attracted to the surface charge via the Coulomb force, electrically screening the first layer. This second layer is loosely associated with the object. It is made of free ions that move in the fluid under the influence of electric attraction and thermal motion rather than being firmly anchored. It is thus called the "diffuse layer".
A Pickering emulsion is an emulsion that is stabilized by solid particles which adsorb onto the interface between the two phases. This type of emulsion was named after S.U. Pickering, who described the phenomenon in 1907, although the effect was first recognized by Walter Ramsden in 1903.
Peptization or deflocculation is the process of converting precipitate into colloid by shaking it with a suitable electrolyte called peptizing agent.
Bacterial adhesion involves the attachment of bacteria on the surface. This interaction plays an important role in natural system as well as in environmental engineering. The attachment of biomass on the membrane surface will result in membrane fouling, which can significantly reduce the efficiency of the treatment system using membrane filtration process in wastewater treatment plants. The low adhesion of bacteria to soil is essential key for the success of in-situ bioremediation in groundwater treatment. However, the contamination of pathogens in drinking water could be linked to the transportation of microorganisms in groundwater and other water sources. Controlling and preventing the adverse impact of the bacterial deposition on the aquatic environment need a deeply understanding about the mechanisms of this process. DLVO theory has been used extensively to describe the deposition of bacteria in many current researches.
Mucoadhesion describes the attractive forces between a biological material and mucus or mucous membrane. Mucous membranes adhere to epithelial surfaces such as the gastrointestinal tract (GI-tract), the vagina, the lung, the eye, etc. They are generally hydrophilic as they contain many hydrogen macromolecules due to the large amount of water within its composition. However, mucin also contains glycoproteins that enable the formation of a gel-like substance. Understanding the hydrophilic bonding and adhesion mechanisms of mucus to biological material is of utmost importance in order to produce the most efficient applications. For example, in drug delivery systems, the mucus layer must be penetrated in order to effectively transport micro- or nanosized drug particles into the body. Bioadhesion is the mechanism by which two biological materials are held together by interfacial forces.
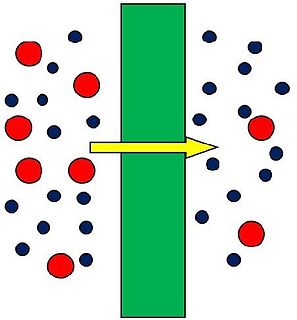
A membrane is a selective barrier; it allows some things to pass through but stops others. Such things may be molecules, ions, or other small particles. Biological membranes include cell membranes ; nuclear membranes, which cover a cell nucleus; and tissue membranes, such as mucosae and serosae. Synthetic membranes are made by humans for use in laboratories and industry.
Adsorption of polyelectrolytes on solid substrates is a surface phenomenon where long-chained polymer molecules with charged groups bind to a surface that is charged in the opposite polarity. On the molecular level, the polymers do not actually bond to the surface, but tend to "stick" to the surface via intermolecular forces and the charges created by the dissociation of various side groups of the polymer. Because the polymer molecules are so long, they have a large amount of surface area with which to contact the surface and thus do not desorb as small molecules are likely to do. This means that adsorbed layers of polyelectrolytes form a very durable coating. Due to this important characteristic of polyelectrolyte layers they are used extensively in industry as flocculants, for solubilization, as supersorbers, antistatic agents, as oil recovery aids, as gelling aids in nutrition, additives in concrete, or for blood compatibility enhancement to name a few.
Protein adsorption refers to the adhesion of proteins to solid surfaces. This phenomenon is an important issue in the food processing industry, particularly in milk processing and wine and beer making. Excessive adsorption, or protein fouling, can lead to health and sanitation issues, as the adsorbed protein is very difficult to clean and can harbor bacteria, as is the case in biofilms. Product quality can be adversely affected if the adsorbed material interferes with processing steps, like pasteurization. However, in some cases protein adsorption is used to improve food quality, as is the case in fining of wines.

Double layer forces occur between charged objects across liquids, typically water. This force acts over distances that are comparable to the Debye length, which is on the order of one to a few tenths of nanometers. The strength of these forces increases with the magnitude of the surface charge density. For two similarly charged objects, this force is repulsive and decays exponentially at larger distances, see figure. For unequally charged objects and eventually at shorted distances, these forces may also be attractive. The theory due to Derjaguin, Landau, Verwey, and Overbeek (DLVO) combines such double layer forces together with Van der Waals forces in order to estimate the actual interaction potential between colloidal particles.
A depletion force is an effective attractive force that arises between large colloidal particles that are suspended in a dilute solution of depletants, which are smaller solutes that are preferentially excluded from the vicinity of the large particles. One of the earliest reports of depletion forces that lead to particle coagulation is that of Bondy, who observed the separation or 'creaming' of rubber latex upon addition of polymer depletant molecules to solution. More generally, depletants can include polymers, micelles, osmolytes, ink, mud, or paint dispersed in a continuous phase.