
A colloid is a mixture in which one substance consisting of microscopically dispersed insoluble particles is suspended throughout another substance. Some definitions specify that the particles must be dispersed in a liquid, while others extend the definition to include substances like aerosols and gels. The term colloidal suspension refers unambiguously to the overall mixture. A colloid has a dispersed phase and a continuous phase. The dispersed phase particles have a diameter of approximately 1 nanometre to 1 micrometre.

A gel is a semi-solid that can have properties ranging from soft and weak to hard and tough. Gels are defined as a substantially dilute cross-linked system, which exhibits no flow when in the steady state, although the liquid phase may still diffuse through this system.

A micelle or micella is an aggregate of surfactant amphipathic lipid molecules dispersed in a liquid, forming a colloidal suspension. A typical micelle in water forms an aggregate with the hydrophilic "head" regions in contact with surrounding solvent, sequestering the hydrophobic single-tail regions in the micelle centre.
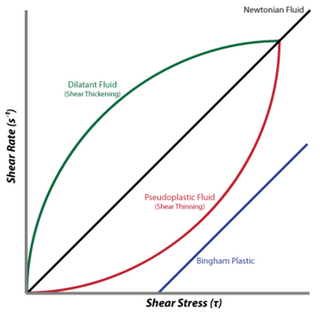
A dilatant material is one in which viscosity increases with the rate of shear strain. Such a shear thickening fluid, also known by the initialism STF, is an example of a non-Newtonian fluid. This behaviour is usually not observed in pure materials, but can occur in suspensions.
In physical chemistry, the Derjaguin–Landau–Verwey–Overbeek (DLVO) theory explains the aggregation and kinetic stability of aqueous dispersions quantitatively and describes the force between charged surfaces interacting through a liquid medium. It combines the effects of the van der Waals attraction and the electrostatic repulsion due to the so-called double layer of counterions. The electrostatic part of the DLVO interaction is computed in the mean field approximation in the limit of low surface potentials - that is when the potential energy of an elementary charge on the surface is much smaller than the thermal energy scale, . For two spheres of radius each having a charge separated by a center-to-center distance in a fluid of dielectric constant containing a concentration of monovalent ions, the electrostatic potential takes the form of a screened-Coulomb or Yukawa potential,

Polyelectrolytes are polymers whose repeating units bear an electrolyte group. Polycations and polyanions are polyelectrolytes. These groups dissociate in aqueous solutions (water), making the polymers charged. Polyelectrolyte properties are thus similar to both electrolytes (salts) and polymers and are sometimes called polysalts. Like salts, their solutions are electrically conductive. Like polymers, their solutions are often viscous. Charged molecular chains, commonly present in soft matter systems, play a fundamental role in determining structure, stability and the interactions of various molecular assemblies. Theoretical approaches to describe their statistical properties differ profoundly from those of their electrically neutral counterparts, while technological and industrial fields exploit their unique properties. Many biological molecules are polyelectrolytes. For instance, polypeptides, glycosaminoglycans, and DNA are polyelectrolytes. Both natural and synthetic polyelectrolytes are used in a variety of industries.
A surface charge is an electric charge present on a two-dimensional surface. These electric charges are constrained on this 2-D surface, and surface charge density, measured in coulombs per square meter (C•m−2), is used to describe the charge distribution on the surface. The electric potential is continuous across a surface charge and the electric field is discontinuous, but not infinite; this is unless the surface charge consists of a dipole layer. In comparison, the potential and electric field both diverge at any point charge or linear charge.

Particle agglomeration refers to the formation of assemblages in a suspension and represents a mechanism leading to the functional destabilization of colloidal systems. During this process, particles dispersed in the liquid phase stick to each other, and spontaneously form irregular particle assemblages, flocs, or agglomerates. This phenomenon is also referred to as coagulation or flocculation and such a suspension is also called unstable. Particle agglomeration can be induced by adding salts or other chemicals referred to as coagulant or flocculant.
Protein precipitation is widely used in downstream processing of biological products in order to concentrate proteins and purify them from various contaminants. For example, in the biotechnology industry protein precipitation is used to eliminate contaminants commonly contained in blood. The underlying mechanism of precipitation is to alter the solvation potential of the solvent, more specifically, by lowering the solubility of the solute by addition of a reagent.
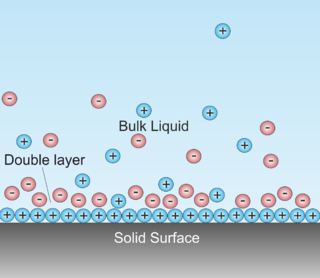
In surface science, a double layer is a structure that appears on the surface of an object when it is exposed to a fluid. The object might be a solid particle, a gas bubble, a liquid droplet, or a porous body. The DL refers to two parallel layers of charge surrounding the object. The first layer, the surface charge, consists of ions which are adsorbed onto the object due to chemical interactions. The second layer is composed of ions attracted to the surface charge via the Coulomb force, electrically screening the first layer. This second layer is loosely associated with the object. It is made of free ions that move in the fluid under the influence of electric attraction and thermal motion rather than being firmly anchored. It is thus called the "diffuse layer".
Layer-by-layer (LbL) deposition is a thin film fabrication technique. The films are formed by depositing alternating layers of complementary materials with wash steps in between. This can be accomplished by using various techniques such as immersion, spin, spray, electromagnetism, or fluidics.
The peptization of a liquid mixture is the process of converting the mixture into a colloid by shaking it with a suitable electrolyte called a peptizing agent. That is, the insoluble solid particles which have settled out of the mixture are reformed into microscopic particles suspended in the mixture. Peptization is the reverse of flocculation, the aggregation of colloidal particles into precipitate; as such, it is also known as deflocculation.
Paint has four major components: pigments, binders, solvents, and additives. Pigments serve to give paint its color, texture, toughness, as well as determining if a paint is opaque or not. Common white pigments include titanium dioxide and zinc oxide. Binders are the film forming component of a paint as it dries and affects the durability, gloss, and flexibility of the coating. Polyurethanes, polyesters, and acrylics are all examples of common binders. The solvent is the medium in which all other components of the paint are dissolved and evaporates away as the paint dries and cures. The solvent also modifies the curing rate and viscosity of the paint in its liquid state. There are two types of paint: solvent-borne and water-borne paints. Solvent-borne paints use organic solvents as the primary vehicle carrying the solid components in a paint formulation, whereas water-borne paints use water as the continuous medium. The additives that are incorporated into paints are a wide range of things which impart important effects on the properties of the paint and the final coating. Common paint additives are catalysts, thickeners, stabilizers, emulsifiers, texturizers, biocides to fight bacterial growth, etc.
Adsorption is the accumulation and adhesion of molecules, atoms, ions, or larger particles to a surface, but without surface penetration occurring. The adsorption of larger biomolecules such as proteins is of high physiological relevance, and as such they adsorb with different mechanisms than their molecular or atomic analogs. Some of the major driving forces behind protein adsorption include: surface energy, intermolecular forces, hydrophobicity, and ionic or electrostatic interaction. By knowing how these factors affect protein adsorption, they can then be manipulated by machining, alloying, and other engineering techniques to select for the most optimal performance in biomedical or physiological applications.
Polymeric materials have widespread application due to their versatile characteristics, cost-effectiveness, and highly tailored production. The science of polymer synthesis allows for excellent control over the properties of a bulk polymer sample. However, surface interactions of polymer substrates are an essential area of study in biotechnology, nanotechnology, and in all forms of coating applications. In these cases, the surface characteristics of the polymer and material, and the resulting forces between them largely determine its utility and reliability. In biomedical applications for example, the bodily response to foreign material, and thus biocompatibility, is governed by surface interactions. In addition, surface science is integral part of the formulation, manufacturing, and application of coatings.
Silanization of silicon and mica is the coating of these materials with a thin layer of self assembling units.
Polyelectrolytes are charged polymers capable of stabilizing colloidal emulsions through electrostatic interactions. Their effectiveness can be dependent on molecular weight, pH, solvent polarity, ionic strength, and the hydrophilic-lipophilic balance (HLB). Stabilized emulsions are useful in many industrial processes, including deflocculation, drug delivery, petroleum waste treatment, and food technology.

Double layer forces occur between charged objects across liquids, typically water. This force acts over distances that are comparable to the Debye length, which is on the order of one to a few tenths of nanometers. The strength of these forces increases with the magnitude of the surface charge density. For two similarly charged objects, this force is repulsive and decays exponentially at larger distances, see figure. For unequally charged objects and eventually at shorted distances, these forces may also be attractive. The theory due to Derjaguin, Landau, Verwey, and Overbeek (DLVO) combines such double layer forces together with Van der Waals forces in order to estimate the actual interaction potential between colloidal particles.

Bovine submaxillary mucin (BSM) coatings are a surface treatment provided to biomaterials intended to reduce the growth of disadvantageous bacteria and fungi such as S. epidermidis, E. coli, and Candida albicans. BSM is a substance extracted from the fresh salivary glands of cows. It exhibits unique physical properties, such as high molecular weight and amphiphilicity, that allow it to be used for many biomedical applications.
Polymer soil stabilization refers to the addition of polymers to improve the physical properties of soils, most often for geotechnical engineering, construction, or agricultural projects. Even at very small concentrations within soils, various polymers have been shown to increase water retention and reduce erosion, increase soil shear strength, and support soil structure. A wide range of polymers have been used to address problems ranging from the prevention of desertification to the reinforcement of roadbeds.



















