
The pnictogens are the chemical elements in group 15 of the periodic table. This group is also known as the nitrogen group or nitrogen family. Group 15 consists of the elements nitrogen (N), phosphorus (P), arsenic (As), antimony (Sb), bismuth (Bi), and moscovium (Mc).

Stibnite, sometimes called antimonite, is a sulfide mineral with the formula Sb2S3. This soft grey material crystallizes in an orthorhombic space group. It is the most important source for the metalloid antimony. The name is derived from the Greek στίβι stibi through the Latin stibium as the former name for the mineral and the element antimony.

Murdochite is a mineral combining lead and copper oxides with the chemical formula PbCu
6O
8−x(Cl,Br)
2x (x ≤ 0.5).

Valentinite is an antimony oxide mineral with formula Sb2O3. Valentinite crystallizes in the orthorhombic system and typically forms as radiating clusters of euhedral crystals or as fibrous masses. It is colorless to white with occasional shades or tints of yellow and red. It has a Mohs hardness of 2.5 to 3 and a specific gravity of 5.76. Valentinite occurs as a weathering product of stibnite and other antimony minerals. It is dimorphous with the isometric antimony oxide senarmontite.

Kermesite or antimony oxysulfide is also known as red antimony or purpur blende (Sb2S2O). The mineral's color ranges from cherry red to a dark red to a black. Kermesite is the result of partial oxidation between stibnite (Sb2S3) and other antimony oxides such as valentinite (Sb2O3) or stibiconite (Sb3O6(OH)). Under certain conditions with oxygenated fluids the transformation of all sulfur to oxygen would occur but kermesite occurs when that transformation is halted.

Jerrygibbsite is a rare silicate mineral with the chemical formula (Mn,Zn)9(SiO4)4(OH)2. Jerrygibbsite was originally discovered by Pete J. Dunn in 1984, who named it after mineralogist Gerald V. Gibbs. It has only been reported from the type locality of Franklin Furnace, New Jersey, United States, and in Namibia's Otjozondjupa region. Jerrygibbsite is member of the leucophoenite family of the humite group. It is always found with these two minerals. It is a dimorph of sonolite.

Stibiconite is an antimony oxide mineral with formula: Sb3O6(OH). Its name originates from Greek stíbi (στίβι), 'antimony' and kónis (κόνις), 'powder', alluding to its composition and habit. It is a member of the pyrochlore super group.

Alacránite (As8S9) is an arsenic sulfide mineral first discovered in the Uzon caldera, Kamchatka, Russia. It was named for its occurrence in the Alacrán silver/arsenic/antimony mine. Pampa Larga, Chile. It is generally more rare than realgar and orpiment. Its origin is hydrothermal. It occurs as subhedral to euhedral tabular orange to pale gray crystals that are transparent to translucent. It has a yellow-orange streak with a hardness of 1.5. It crystallizes in the monoclinic crystal system. It occurs with realgar and uzonite as flattened and prismatic grains up to 0.5 mm across.

Bergenite is a rare uranyl phosphate of the more specific phosphuranylite group. The phosphuranylite-type sheet in bergenite is a new isomer of the group, with the uranyl phosphate tetrahedra varying in an up-up-down, same-same-opposite (uuduudSSOSSO) orientation. All bergenite samples have been found in old mine dump sites. Uranyl minerals are a large constituent of uranium deposits.
Xikuangshan mine (simplified Chinese: 锡矿山; traditional Chinese: 錫礦山; pinyin: Xīkuàngshān) in Lengshuijiang, Hunan, China, contains the world's largest known deposit of antimony. It is unique in that there is a large deposit of stibnite (Sb2S3) in a layer of Devonian limestone. There are three mineral beds which are between 2.5 and 8 m thick which are folded in an anticline that plunges to the south-west. The total mineralised area of the mine has a surface extent of 14 km2. There are two different units at the mine, the northern one produces mixed oxide and sulfide such as stibiconite (Sb3O6(OH)) and the southern one produces stibnite. Ore is concentrated and refined on site in a refinery with a capacity of 10,000 tonnes of antimony per year.
Penikisite was discovered by Alan Kulan and Gunar Penikis near Rapid Creek, Yukon Territory. The mineral is a member of the bjarebyite group along with kulanite, ideally BaFe2+2Al2(PO4)3(OH)3, and bjarebyite, ideally BaMn2+2Al2(PO4)3(OH)3. It is among several new minerals that have been discovered in the Rapid Creek and Big Fish areas of Yukon Territory. Kulanite is similar in many ways to penikisite in appearance and properties. The chemical formula for penikisite is Ba(Mg,Fe,Ca)Al2(PO4)2(OH)3. It has a hardness of about 4 and a density of 3.79 g/cm3. Penikisite is unique among the bjarebyite group in being monoclinic and has a biaxial optical class. It comes in shades of blue and green and, when rubbed on a streak plate, is pale green to white in color. Although penikisite and kulanite both range from blue to green, penikisite zones are easily distinguishable from kulanite zones in kulanite-penikisite crystals because they are lighter than the darker kulanite in color. Penikisite is a phosphate and is different from kulanite in that it is a magnesium-rich phosphate whereas kulanite is an iron-rich phosphate.
Eveslogite is a complex inosilicate mineral with a chemical formula (Ca,K,Na,Sr,Ba)
48[(Ti,Nb,Fe,Mn)
12(OH)
12Si
48O
144](F,OH,Cl)
14 found on Mt. Eveslogchorr in Khibiny Mountains, on the Kola peninsula, Russia. It was named after the place it was found. This silicate mineral occurs as an anchimonomineral veinlet that cross-cuts poikilitic nepheline syenite. This mineral appears to resemble yuksporite, as it forms similar placated fine fibrous of approximately 0.05 to 0.005mm that aggregates outwardly. The color of eveslogite is yellow or rather light brown. In addition, it is a semitransparent mineral that has a white streak and a vitreous luster. Its crystal system is monoclinic and possesses a hardness (Mohs) of 5. This newly discovered mineral belongs to the astrophyllite group of minerals and contains structures that are composed of titanosilicate layers. Limited information about this mineral exists due to the few research studies carried out since its recent discovery.
Scotlandite is a sulfite mineral first discovered in a mine at Leadhills in South Lanarkshire, Scotland, an area known to mineralogists and geologists for its wide range of different mineral species found in the veins that lie deep in the mine shafts. This specific mineral is found in the Susanna vein of Leadhills, where the crystals are formed as chisel-shaped or bladed. Scotlandite was actually the first naturally occurring sulfite, which has the ideal chemical formula of PbSO3. The mineral has been approved by the Commission on New Minerals and Mineral Names, IMA, to be named scotlandite for Scotland.
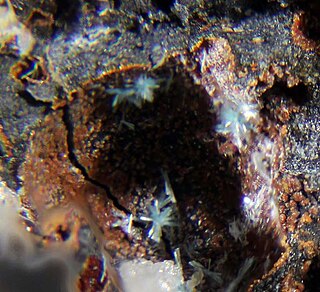
Mammothite is a mineral found in the Mammoth mine in Tiger, Arizona and also in Laurium, Attika, Greece. This mineral was named in 1985 by Donald R. Peacor, Pete J. Dunn, G. Schnorrer-Köhler, and Richard A. Bideaux, for the Mammoth vein (one of the two main veins in the mine) and the town of Mammoth, Arizona, which was named for the mine. The mammothite that is found in Arizona exist as euhedral crystals imbedded in micro granular, white colored anglesite with a saccharoidal texture. The associated minerals include phosgenite, wulfenite, leadhillite and caledonite. In Greece, the mammothite exists as small euhedral crystals and also as microscopic rock cavities lined with projecting crystals within the slags. The associated minerals here are cerussite, phosgenite and matlockite. The ideal chemical formula for mammothite is Pb6Cu4AlSb5+O2(OH)16Cl4(SO4)2.
Lahnsteinite is a basic sulfate mineral first discovered in the Friedrichssegen Mine, Germany in a goethite cavity. Though found in goethite, the crystals of Lahnsteinite are few millimeters in size, and are tabular shaped. Lahnsteinite was the first mineral discovered in the Lahn Valley deposits. The empirical formula for lahnsteinite is (Zn3.3,Fe0.27,Cu0.11)3.91(S0.98O4)(OH)5*3H2.10O.
Vanarsite, NaCa12(As3+V4+3.5V5+8.5As5+6O51)2・78H2O, is a mineral that forms as very dark blue blades that are flattened on {100} and elongated on [010]. The crystals form in sub-parallel intergrowths in aggregates up to 5 mm long and are part of the cubic crystal system. The mineral is found in the Packrat mine in Colorado, USA in association with three mineral of similar composition, packratite, Ca11(As3+V4+2V5+10As5+6O51)2・83H2O, morrisonite, Ca6(As3+V4+2V5+10As5+6O51)2・78H2O, and gatewayite, Ca11(As3+V4+3V5+9As5+6O51)・31H2O. Vanarsite has been determined to be part of the vanadate, arsenite, and arsenate groups. Analytical methods were used to determine its chemical composition, physical properties, and structure.
Hexahydroborite is a mineral composed of calcium, boron, oxygen, and hydrogen, with formula CaB2H12O6, more precisely [Ca2+]([B(OH4)]−)2·2H2O.
Takedaite is a borate mineral that was found in a mine in Fuka, Okayama Prefecture Japan during a mineralogical survey in the year 1994. During the survey, Kusachi and Henmi reported the occurrence of an unidentified anhydrous borate mineral closely associated with nifontovite, olshanskyite, and calcite. By the year 1994 two other minerals in the borate group M3B2O6 had been identified in nature Mg3B2O6 known as kotoite and Mn3B2O6 known as jimboite. Takedaite has the ideal chemical formula of Ca3B2O6. The mineral has been approved by the Commission on New Minerals and Mineral Names, IMA, to be named takedaite after Hiroshi Takeda, a professor at the Mineralogical Institute, University of Tokyo Japan.
Daliranite is a sulfosalt found in northwestern Iran with a general chemical formula of PbHgAs2S5. The mineral presents a vibrant orange-red color and fibrous habit which makes it resemble the oxide ludlockite being confused by its similarities in early studies. Named after Dr. Farahnaz Daliran, who has important contributions to research on ore deposits in Iran, this mineral was accepted by the International Mineralogical Association (IMA) in 2007.
Ophirite is a tungstate mineral first discovered in the Ophir Hill Consolidated mine at Ophir district, Oquirrh Mountains, Tooele County, Utah, United States of America. It was found underground near a calcite cave in one veinlet, six centimeters wide by one meter long, surrounded by different sulfides. Before the closing of the mine in 1972, it was dominated by sulfide minerals, and the Ophir district was known for being a source of zinc, copper, silver, and lead ores. The crystals are formed as tablets. It is the first known mineral to contain a heteropolyanion, a lacunary defect derivative of the Keggin anion. The chemical formula of ophirite is Ca2Mg4[Zn2Mn3+2(H2O)2(Fe3+W9O34)2] · 46•H2O. The mineral has been approved by the Commission on New Minerals and Mineral Names, IMA, to be named ophirite for its type locality, the Ophir Consolidated mine.








