
Glycolysis is the metabolic pathway that converts glucose into pyruvate, and in most organisms, occurs in the liquid part of cells, the cytosol. The free energy released in this process is used to form the high-energy molecules adenosine triphosphate (ATP) and reduced nicotinamide adenine dinucleotide (NADH). Glycolysis is a sequence of ten reactions catalyzed by enzymes.

Pseudoephedrine (PSE) is a sympathomimetic drug of the phenethylamine and amphetamine chemical classes. It may be used as a nasal/sinus decongestant, as a stimulant, or as a wakefulness-promoting agent in higher doses.

Cellular respiration is the process by which biological fuels are oxidised in the presence of an inorganic electron acceptor, such as oxygen, to drive the bulk production of adenosine triphosphate (ATP), which contains energy. Cellular respiration may be described as a set of metabolic reactions and processes that take place in the cells of organisms to convert chemical energy from nutrients into ATP, and then release waste products.

Anabolism is the set of metabolic pathways that construct molecules from smaller units. These reactions require energy, known also as an endergonic process. Anabolism is the building-up aspect of metabolism, whereas catabolism is the breaking-down aspect. Anabolism is usually synonymous with biosynthesis.

Polyhydroxyalkanoates or PHAs are polyesters produced in nature by numerous microorganisms, including through bacterial fermentation of sugars or lipids. When produced by bacteria they serve as both a source of energy and as a carbon store. More than 150 different monomers can be combined within this family to give materials with extremely different properties. These plastics are biodegradable and are used in the production of bioplastics.
Shikimic acid, more commonly known as its anionic form shikimate, is a cyclohexene, a cyclitol and a cyclohexanecarboxylic acid. It is an important biochemical metabolite in plants and microorganisms. Its name comes from the Japanese flower shikimi, from which it was first isolated in 1885 by Johan Fredrik Eykman. The elucidation of its structure was made nearly 50 years later.
Industrial fermentation is the intentional use of fermentation in manufacturing processes. In addition to the mass production of fermented foods and drinks, industrial fermentation has widespread applications in chemical industry. Commodity chemicals, such as acetic acid, citric acid, and ethanol are made by fermentation. Moreover, nearly all commercially produced industrial enzymes, such as lipase, invertase and rennet, are made by fermentation with genetically modified microbes. In some cases, production of biomass itself is the objective, as is the case for single-cell proteins, baker's yeast, and starter cultures for lactic acid bacteria used in cheesemaking.

In biochemistry, mixed acid fermentation is the metabolic process by which a six-carbon sugar is converted into a complex and variable mixture of acids. It is an anaerobic (non-oxygen-requiring) fermentation reaction that is common in bacteria. It is characteristic for members of the Enterobacteriaceae, a large family of Gram-negative bacteria that includes E. coli.

Amino acid synthesis is the set of biochemical processes by which the amino acids are produced. The substrates for these processes are various compounds in the organism's diet or growth media. Not all organisms are able to synthesize all amino acids. For example, humans can synthesize 11 of the 20 standard amino acids. These 11 are called the non-essential amino acids).
The Pasteur effect describes how available oxygen inhibits ethanol fermentation, driving yeast to switch toward aerobic respiration for increased generation of the energy carrier adenosine triphosphate (ATP).

The acetolactate synthase (ALS) enzyme is a protein found in plants and micro-organisms. ALS catalyzes the first step in the synthesis of the branched-chain amino acids.
Glucose-1,6-bisphosphate synthase is a type of enzyme called a phosphotransferase and is involved in mammalian starch and sucrose metabolism. It catalyzes the transfer of a phosphate group from 1,3-bisphosphoglycerate to glucose-1-phosphate, yielding 3-phosphoglycerate and glucose-1,6-bisphosphate.

Cystathionine beta-lyase, also commonly referred to as CBL or β-cystathionase, is an enzyme that primarily catalyzes the following α,β-elimination reaction
The enzyme methylglyoxal synthase catalyzes the chemical reaction

In enzymology, a malate synthase (EC 2.3.3.9) is an enzyme that catalyzes the chemical reaction
2-Succinyl-6-hydroxy-2,4-cyclohexadiene-1-carboxylate synthase, also known as SHCHC synthase is encoded by the menH gene in Escherichia coli and functions in the synthesis of vitamin K. The specific step in the synthetic pathway that SHCHC synthase catalyzes is the conversion of 5-enolpyruvoyl-6-hydroxy-2-succinylcyclohex-3-ene-1-carboxylate to (1R,6R)-6-hydroxy-2-succinylcyclohexa-2,4-diene-1-carboxylate and pyruvate.

Cofactor engineering, a subset of metabolic engineering, is defined as the manipulation of the use of cofactors in an organism’s metabolic pathways. In cofactor engineering, the concentrations of cofactors are changed in order to maximize or minimize metabolic fluxes. This type of engineering can be used to optimize the production of a metabolite product or to increase the efficiency of a metabolic network. The use of engineering single celled organisms to create lucrative chemicals from cheap raw materials is growing, and cofactor engineering can play a crucial role in maximizing production. The field has gained more popularity in the past decade and has several practical applications in chemical manufacturing, bioengineering and pharmaceutical industries.
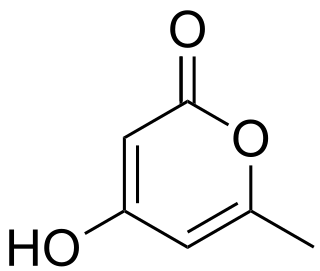
Triacetic acid lactone is an organic compound derived enzymatically from glucose. It is a light yellow solid that is soluble in organic solvents.
4-Hydroxy-tetrahydrodipicolinate synthase (EC 4.3.3.7, dihydrodipicolinate synthase, dihydropicolinate synthetase, dihydrodipicolinic acid synthase, L-aspartate-4-semialdehyde hydro-lyase (adding pyruvate and cyclizing), dapA (gene)) is an enzyme with the systematic name L-aspartate-4-semialdehyde hydro-lyase (adding pyruvate and cyclizing; (4S)-4-hydroxy-2,3,4,5-tetrahydro-(2S)-dipicolinate-forming). This enzyme catalyses the following chemical reaction

Industrial microbiology is a branch of biotechnology that applies microbial sciences to create industrial products in mass quantities, often using microbial cell factories. There are multiple ways to manipulate a microorganism in order to increase maximum product yields. Introduction of mutations into an organism may be accomplished by introducing them to mutagens. Another way to increase production is by gene amplification, this is done by the use of plasmids, and vectors. The plasmids and/ or vectors are used to incorporate multiple copies of a specific gene that would allow more enzymes to be produced that eventually cause more product yield. The manipulation of organisms in order to yield a specific product has many applications to the real world like the production of some antibiotics, vitamins, enzymes, amino acids, solvents, alcohol and daily products. Microorganisms play a big role in the industry, with multiple ways to be used. Medicinally, microbes can be used for creating antibiotics in order to treat infection. Microbes can also be used for the food industry as well. Microbes are very useful in creating some of the mass produced products that are consumed by people. The chemical industry also uses microorganisms in order to synthesize amino acids and organic solvents. Microbes can also be used in an agricultural application for use as a biopesticide instead of using dangerous chemicals and or inoculants to help plant proliferation.















